![]()
Effective treatment strategies against Ebola virus
Amina Yaqoob*, Umara Shehzad, Zarnab Ahmad, Nadia Naseer, Saliha Bashir
Adv. life sci., vol. 2, no. 4, pp. 176-182, August 2015
*- Corresponding Author: Amina Yaqoob (Email: amina.yaqoob@cemb.edu.pk)
Authors Affiliations[Date Received: 26/05/2015; Date Revised: 22/08/2015; Date Published Online: 25/08/2015]
Abstract![]()
Introduction
Methods
Discussion
Conclusion
References
Abstract
Ebola virus (EBOV), a member of order Mononegavirales is most famous for causing the endemics of hemorrhagic fever in different countries of the world. Various effective treatment for EBOV are available presently but different clinical trials and experimental studies on animal models are ongoing for this purpose. Results from different studies showed that selective vaccines and therapeutic drugs have potential to interfere the viral life events within host cell in order to inhibit its replication. Various pre-clinical trials in this regard are proved successful on non-human primates (NHPs) and found to be significant in inhibiting EBOV infections. It is the need of hour to develop effective vaccines against Ebola virus to combat this problem as soon as possible. The present article is a brief review on potential treatment strategies against Ebola virus.
Keywords: Filovirus, Ebola hemorrhagic fever, vaccines, antivirals, clinical trials
Introduction
Ebola virus (EBOV) belongs to the family filoviridae (Negative sense RNA) in the order of mononegavirales and is associated with severe type of hemorrhagic fever. The fever leads to mortality rate of 90% in human and 100% in non-human primates [1, 2]. Five species of EBOV are Bundibugyo ebolavirus, Reston ebolavirus, Sudan ebolavirus, Zaire ebolavirus and Tai Forest ebolavirus. Since 1970 after the identification of Zaire Ebola virus, almost 20 outbreaks of EBOV have been stated in Central Africa [3]. Manifested clinical symptoms of its infection include variable types of hemorrhages, fever hypotension, disorders of coagulation, widespread destruction of the focal tissue, shocks, difficulties in fluid distribution within the body and multi organ failure [4, 5]. The mature EBOV particle consists of internal helical structure, nucleocapsid, a unit membrane envelope and a surface projection layer of GP spikes. GP spikes are the outer projection of envelope, they are necessary for the viral attachment to the membrane of target cell [6, 7]. The viral genome consists of seven genes that are arranged in a sequence of 3′ leader-NP-VP35-VP40-GP-VP30-VP24-RNA polymerase ‘L’.
The EBOV infection starts from the small lesion that can spread to other body parts via lymphatic or vascular systems directly. Primary infected organs are the lymph nodes, spleen and liver [8]. However mononuclear phagocytic cells are primary targeting cells for viral replication [9, 10].EBOV causes a partial dysfunction of these cells and ultimately, pro-inflammatory mediators and vasoactive substances are released largely at the site of infection. The release of these substances and accumulation of other target cells and distribution of viral proteins into tissue macrophages or dendritic cells throughout the body leads to more severe infection [10, 11]. Eventually, one can say that Ebola virus pathogenesis is basically the impairment of immune system [2, 12-17].
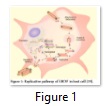
Major steps in viral replication within host cell involve the interaction of virus to the cell surface receptor followed by endocytosis or endosome formation in host cell. The viral envelope fuses with host endosome membrane and as a result, viral particle releases into cell’s cytoplasm. After viral replication using host’s replicative machinery, enveloped viral particles bud out of the cell with the help of VP40 (Figure 1). High degree polyploidy of EBOV is due to the polymorphism in viral budding property. Usually Filoviruses show more extensive polyploidy as compared to any group of viruses [18].
Despite the significant knowledge about Ebola hemorrhagic fever, advancements in developing therapeutic strategies against this lethal infection has been much slower. The editorial has discussed various significant therapeutics which are experimentally considered to be the potent interfering agents in the life events of EBOV.
Methods
Search strategy and literature selection criteria
The present article has been summarized by reviewing 45 articles related to EBOV infection and target drugs against its infection. Whole of the present data has collected from experimental studies of 2010 to 2015 and the search engines used are Google scholar, NCBI and Pubmed.
Discussion
1. INHIBITORS TO VIRAL ENTRY INTO HOST CELLS
Most important step in Ebola virus life cycle is its entry into the host cell. The major steps involve in EBOV entry are binding to the host cell through the interaction between viral glycoprotein (GP) and host cell membrane receptors, entry of virus, formation of viral endosome, internalization of virus or release of virus particle into host cell’s cytoplasm [20].
SERMs; A strong, inhibition of Ebola virus infection has been estimated in vitro by selective estrogen receptor modulators (SERMs) i.e. toremiphene and clomiphene. These are FDA (Food and Drugs Authority) approved drugs which inhibit EBOV infection through targeting the viral entry into host cell [21]. Their activity against this lethal infection was confirmed in an in vivo mouse infection model where the dosage of toremiphene and clomiphene (60 mg/kg) to affected mice produced rates of survival 90% and 50% respectively [22]. SERMs blocked viral entry after internalization and interfere with a step later in entry of virus where they affect the triggering of fusion [23]. It clearly depicted they did not work by classical pathways i.e. independent of estrogen receptors (ERs), however their activity is affected by over expression of Niemann-Pick C1 (NPC1), the endosomal membrane protein which binds to the viral GP [24].
Ion channel blockers; Verapamil, dronedarone and amiodarone are clinically approved related antiviral drugs that have similar mode of action against Filoviruses. These are ion channel blockers and retard Filoviral entry into the target cells [25].
2. ANTIVIRALS TARGETING EBOV REPLICATION
Following the uncoating viral genome is exposed to host replication machinery. Polymerase L and VP35 constitute polymerase complex and transcribe viral mRNA that is read by cellular ribosomes and produce viral protein. mRNA that is produced in this process have positive polarity so it acts as template for negative sense mRNA synthesis [26].
Lipid nanoparticle small interfering RNA (LNP-siRNA) work against viral replication by slowing it down. Experimental studies have manifested that small interfering RNAs are more effectual in the treatment of EBOV infections when compared with phosphorodiamidate morpholino oligomers (PMOs) which are mRNA translation inhibitors while siRNA degrades the viral genome through RISC (RNA induced silencing complex). PMO is also observed to be quiet unstable in vivo due to nuclease digestion. LNP-siRNA agents have been approved for phase I clinical trials by the FDA for treating EHF [27].
BCX4430; Studies have shown that BCX4430 is a novel and synthetic analogue of adenosine and has broad spectrum antiviral activity in human cells against Filovirus [28]. After exposure to EBOV, intramuscular administration of BCX4430 provided protection in rodent models and they were found to be completely protected from EBOV infection when BCX4430 was administered within 48 hours after infection [28]. Likewise, another in vivo trial on EBOV infected mice, showed that an early oral and intramuscular (twice-daily) dose of BCX4430 led to 100% and 90% survival, respectively. The molecular studies showed that it quickly metabolizes to its derivative 5′-monophosphate, when incorporated into viral RNA results in non-obligate RNA chain termination but not affects the human RNA or DNA [28]. Ames assay revealed that BCX4430 without any substantial mutagenicity, acts against EBOV in vitro and other multiple negative-sense RNA viruses. Further studies are ongoing evaluating the BCX4430 protection from EBOV in nonhuman primates [21].
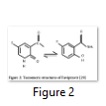
T-705 or Favipiravir; Keeping in view the interferon therapy, Favipiravir is structurally a close derivative of ribavirin. It can be taken as a more specific form of ribavirin that, when activated, acts as an RNA polymerase inhibitor by competitive inhibition in direct competition with GTP [29]. Broad spectrum activity of Favipiravir is due to its different tautomeric forms (as shown in Figure 2).
This drug primarily act as selective inhibitor for the viral replication by inhibiting the activity of RNA-dependent RNA polymerase of virus via producing an active metabolite and also stimulate high frequency of lethal RNA mutation [30]. Astonishing feature of it is activity against (-) RNA viruses i.e. Influenza virus as well as (+) RNA viruses i.e. Flavi and Picornaviruses has been observed and it has been approved for Influenza treatment in Japan since March, 2014 [31].
Experimental study showed that Favipiravir or T-705 has very effective antiviral activity against Filovirus infections when he gave an oral administration of T-705 to an immune-deficient mice and found 100% protection in it. Oestereich et al. indicated that T-705 can also be used as effective drug for inhibiting in vitro EBOV replication without causing any cytotoxicity under the used laboratory conditions [30, 35].
1. SUPPRESSORS TO VIRAL ASSEMBLY AND RELEASE
The final step in viral replication is its assembly and budding out from host cell to further infect the neighboring cells.
Mutant VP40 proteins; The EBOV matrix protein VP40, present under the viral envelope is one of its most abundant matrix proteins that meant for the binding of the viral surface lipids with nucleocapsid. This protein mediates the budding of the virus prior to release from infected cell. Various studies have displayed specific mutations of VP40 to negate viral egress. Up till now, clinical manifestation of VP40 has not been demonstrated. If specific mutations are introduced in VP40 by different molecular strategies, it can interfere with normal functions of virus and prevent systemic infection. [32].
Glycosylation profile (GPs) characterize as most potent element not only for viral maturation but also act as fundamental mediators of viral budding [33]. Various experimental studies have undergone to inhibit EBOV assembly or maturation within host cell by using glycosylation suppressors or declycosylation enzymes. For example, N-linked glycosylation suppressor, tunicamycin when applied on EBOV infected HeLa cells, there is >90% decreased infection [34]. Furthermore imino sugars series (IHVR17028, IHVR11029 and IHVR19029) were presently reported to prevent endoplasmic reticulum (ER) enzyme α-glucosidase I, which is a deglycosylating enzyme and helps in maturation and proper folding of nascent proteins during viral assembly. There is significant increase in survival rate (50–80%) of EBOV infected mice when they were administrated with these three imino sugars [35].
2. EBOLA VACCINATION
There has been a dire need for the development of vaccine for prevention of Ebola virus infections to control the outbreaks and also to fill for the lack of effective treatment of Ebola infection. Many strategies have been employed in the past two decades for the vaccine development against Ebola virus utilizing different animal models. Three different approaches have been used for the vaccine development which differ due to their antigen delivery method. They include non-replicative, expressing vector based vaccines; replication-competent, viral vector based vaccines; and viral protein antigen containing vaccines. The stated approaches have their distinct positive effects and also limitations.
Adenovirus replicon-based recombinant vaccines showed effective protection in non-human primates against Ebola virus infection. The replicons are based on different serotypes and their efficacy has been well studies. Another virus called Venezuelan enquine encephalitis virus (VEEV) has been used for the development of recombinant vaccine. Adenovirus and VEEV replicon based vaccines are limited in their effectiveness due to a pre-existing immunity against them which is a major hindrance to their usage. Kunjin virus, which belongs to the Flaviviridae family and has RNA self-replication ability, has also been used for replicon vaccine development and has shown potential applicability in guinea pig. Newcastle disease virus (NCV) has also been used for recombinant vaccine production. DNA vaccines which express EBOV GP (glycoprotein), in addition to these replicon vaccines, have been investigated and have undergone clinical trials as well. DNA vaccines have an advantage that they can be administered in repetition to enhance the immune response induction.
Reverse genetic technology has been used to produce recombinant vesicular stomatitis virus expressing Ebola virus GP instead of its own glycoprotein VSVG. A derivative form of this vaccine has also been shown to have post-exposure prophylaxix treatment potential of Ebola virus infection. A thorough safety assessment needs to be done as such vaccines are replication competent containing viral vectors in their full viability. Recombinant Rabies virus RABV has also been used for the development of Ebola vaccine. This vaccine has an advantage over the former as it can be used in disabled
form with less safety concerns. Rhabdovirus-based vectors, VSV and RABV, and recombinant paramyxovirus-based vectors for Ebola vaccine development have also been employed. Human parainfluenza virus 3 (HPIV3) with expression for Ebola GP has been tried with non-human primates and is found to be effective. There is an inherent immunity to HPIV3 which reduces the efficacy of this vaccine.
The above stated vaccines, although are found to be almost effectively working in animal models, especially the candidate vaccines effective in non-human primates, but they are replication competent and might raise many safety issues. There also is a problem of already present immunity against the candidate vaccines which dampens the effect of vaccines in subsequent dosage aimed at boosting the immunization. The viral protein antigen based vaccine strategies are safe from these issues. The inactivation protocol followed for this strategy is crucial as it determines the efficiency and efficacy of the vaccines. For instance, Ebola virus inactivated by 1, 5-iodonaphthylazide (INA), a photoinduced alkylating probe, along with UV-irradiation provided full protection in mice, whereas deactivation by gamma irradiation did not have the same effects. The strategy, although very effective, is expensive as it requires Biosafety Level 4 facility for its development.
Purified surface protein and virus-like particles are another category for vaccine development having great potential. Virus-like particles can, on one hand, be repeatedly given to the vaccinated individuals for boosting immune response, and on the other hand, are versatile enough to be manipulated to include different antigenic molecules. They are produced in insect cells so they are comparatively, potentially safe [36].
The vaccines expressing Ebola surface glycoprotein, which have undergone clinical trials include recombinant vesicular stomatitis virus (rVSV-EBOV) vaccine, chimpanzee adenovirus (ChAd3-EBO-Z), human adenovirus (Ad5-EBOV, Ad26-ZEBOV), and vaccinia virus Ankara (MVA-BN Filo), vector aided vaccines, DNA vaccines and/or nanoparticles. Phase 3 trial of rVSV-ZEBOV vaccine is being carried out. This has been a promising candidate in terms of its immunogenicity, but its reactogenecity makes it difficult to be used as a prevalent preventive measure. Reduction in dosage of this vaccine reduces immunogenicity but the risk of viremia and associated symptoms of arthritis, dermatitis and cutaneous vasculitis remains same. Thus more clinical trials and manifestations are required along with thorough testing and optimization [37-39].
1. POST INFECTION THERAPEUTIC OPTIONS
Therapy of Ebola virus infection involves confronting the virus at various fronts. Majority of compounds used in therapy block the virus from entering into the cell or else, aid in prevention of its transcription/replication inside the cell. The mechanism of Ebola virus infection is somewhat similar to the VSV infection, which, due to this reason, serves as a model and a surrogate for EBOV.
Recombinant nematode anticoagulant protein c2; the targets of action of therapeutic agents used in Ebola infection therapy are rNAPc2 (recombinant nematode anticoagulant protein c2) and rhAPC (recombinant human activated protein C) for the treatment of symptomatic coagulopathy and sepsis, siRNAs and monoclonal antibodies for controlling viremia and spread of virus. Use of antibodies as post-exposure prophylaxix has demonstrated protective effect in non-human primates.
Heme-oxygenase I (HO-1) has been considered for the suppression of EBOV transcription/replication in the host cells. Many small molecules which hinder cathepsin L cleavage of viral glycoproteins have been discovered to prevent the entry of EBOV into the host cells. Optimization of these compounds is needed further to develop them as potent antiviral agents. Mice were protected against EBOV infection due to administered chloroquine. This compound has multiple antiviral effects ranging from endocytosis to exocytosis of virus associated particles and also, downregulation of IFN-ɣ and TNF-α production and TNF-α receptors.
All these therapeutic agents need to be extensively studied and optimized as treatment in post infectious conditions. It is difficult and costly to properly carry out studies related to the efficacy of these compounds as BS level 4 labs are required and few labs meet these highest safety levels standards and are equipped to carry out research under this context. Anti VSV therapy could be evaluated as a closely related responsive therapy to EBOV therapy, due to similar viral infectious cycle in both species [40].
Other antivirals; Pegylated interferon has shown antiviral effect against a broad range of viruses. 3-deazaneplanocin A, an S-adenosyl-L-homocysteine (SAH), a hydrolase inhibitor has been found to induce a huge increase in interferon- alpha production in EBOV-infected mice. Griffithsin and similar lectins might be used as antiviral agents as they bind to the terminal mannose residues of asparagine (N)-linked Man 5–9 GlcNAc2 structures which are found on the envelopes of HIV-1, HIV-2, HCV, SARS coronavirus and particularly EBOV.
Inhibition of glycan processing enzymes of host cell leads to misfolded protein entities. Alpha-glucosidase I and II of endoplasmic reticulum are crucial in this regard. CM-10-18, such as IHVR11029, IHVR17028 and IHVR19029 act to suppress the survival of Marburg and Ebola virus infection as recorded in mice. Three compounds FGI-103, FGI-104 and FGI-106 from FGI (Functional Genetics Inc., Gaithersburg, MD), were found to show in vivo antiviral efficacy. NSC62914, an antioxidant, has been found to demonstrate antiviral activity against filovirus, in vitro and in vivo, in mice which have been infected with EBOV or Marburg virus. The fusion of viral membrane with host cellular membrane has been shown to be prevented by a rhodamine derivatives, which are structurally very different compounds. CMLDBU3402 is an RNA transcription inhibitor of EBOV and VSV virus. HSPA5, an endoplasmic reticulum chaperone, has been found to be a target of molecules such as (-)-epigallocatechin gallate [29].
Conclusion
The aggressive infection of Ebola virus has caused large number of deaths in humans as well as NHPs. It is the time to focus on its preventive measure. The current treatments like use of T-705, VP40, BCX4430, LNP-siRNA, SERMs, Heme-oxygenase 1 etc., showed quiet promising result in prevention of EBOV infection but these strategies have two major problems in their use, firstly they are only successful when administered before or just minutes after the onset of viral infection and secondly they are effective only in experimental trials for NHPs. Therefore future studies need to focus on shifting these potential therapeutics into clinical trials. The vaccination and passive immunization methodologies are most effective approaches and must undergo further experimental trials for treatment of Ebola virus infections.
References
- Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nature Reviews Immunology, (2007); 7(7): 556-567.
- Geisbert TW, Hensley LE, Jahrling PB, Larsen T, Geisbert JB, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. The Lancet, (2003); 362(9400): 1953-1958.
- Towner JS, Sealy TK, Khristova ML, Albariño CG, Conlan S, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathogens, (2008); 4(11): e1000212.
- Qiu X, Alimonti JB, Melito PL, Fernando L, Ströher U, et al. Characterization of Zaire ebolavirus glycoprotein-specific monoclonal antibodies. Clinical immunology, (2011); 141(2): 218-227.
- Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Disease Models & Mechanisms, (2009); 2(1-2): 12.
- Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, et al. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature, (2008); 454(7201): 177-182.
- Hood CL, Abraham J, Boyington JC, Leung K, Kwong PD, et al. Biochemical and structural characterization of cathepsin L-processed Ebola virus glycoprotein: implications for viral entry and immunogenicity. Journal of Virology, (2010); 84(6): 2972-2982.
- Leung LW, Park M-S, Martinez O, Valmas C, López CB, et al. Ebolavirus VP35 suppresses IFN production from conventional but not plasmacytoid dendritic cells. Immunology and Cell Biology, (2011); 89(7): 792-802.
- Aronson JF (2014) Viral Hemorrhagic Fevers. Viruses and the Lung: Springer. pp. 123-132.
- Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS neglected tropical diseases, (2010); 4(10): e837.
- Hensley LE, Young HA, Jahrling PB, Geisbert TW. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunology letters, (2002); 80(3): 169-179.
- Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, et al. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Laboratory Investigation, (2000); 80(2): 171-186.
- Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. The American Journal of Pathology, (2003); 163(6): 2347-2370.
- Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, et al. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. Journal of Infectious Diseases, (2003); 188(11): 1618-1629.
- Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. The American Journal of Pathology, (2003); 163(6): 2371-2382.
- Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. Journal of Virology, (2009); 83(14): 7296-7304.
- Prins KC, Delpeut S, Leung DW, Reynard O, Volchkova VA, et al. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. Journal of Virology, (2010); 84(6): 3004-3015.
- Basler CF. Portrait of a killer: genome of the 2014 EBOV outbreak strain. Cell Host & Microbe, (2014); 16(4): 419-421.
- Changula K, Kajihara M, Mweene AS, Takada A. Ebola and Marburg virus diseases in Africa: Increased risk of outbreaks in previously unaffected areas? Microbiology and Immunology, (2014); 58(9): 483-491.
- Gehring G, Rohrmann K, Atenchong N, Mittler E, Becker S, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. Journal of Antimicrobial Chemotherapy, (2014); 69(8): 2123-2131.
- Picazo E, Giordanetto F. Small molecule inhibitors of ebola virus infection. Drug Discovery Today, (2015); 20(2): 277-286.
- Haque A, Hober D, Blondiaux J. Addressing therapeutic options for Ebola virus infection in current or future outbreaks. Antimicrobial Agents and Chemotherapy, (2015); 01105-01115.
- Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, et al. FDA-approved selective estrogen receptor modulators inhibit ebola virus infection. Science Translational Medicine, (2013); 5(190): 190ra179-190ra179.
- Ng M, Ndungo E, Jangra RK, Cai Y, Postnikova E, et al. Cell entry by a novel European filovirus requires host endosomal cysteine proteases and Niemann–Pick C1. Virology, (2014); 468637-646.
- Gehring G, Rohrmann K, Atenchong N, Mittler E, Becker S, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. Journal of Antimicrobial Chemotherapy, (2014); dku091.
- Uebelhoer LS, Albariño CG, McMullan LK, Chakrabarti AK, Vincent JP, et al. High-throughput, luciferase-based reverse genetics systems for identifying inhibitors of Marburg and Ebola viruses. Antiviral Research, (2014); 10686-94.
- Lai KY, Ng WYG, Cheng FF. Human Ebola virus infection in West Africa: a review of available therapeutic agents that target different steps of the life cycle of Ebola virus. Infectious Diseases of Poverty, (2014); 343.
- Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature, (2014); 508(7496): 402-405.
- De Clercq E. Ebola virus (EBOV) infection: Therapeutic strategies. Biochemical Pharmacology, (2015); 93(1): 1-10.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, et al. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Research, (2014); 10517-21.
- Akhtar A, Befkadu E, Basu P, Kumar P. Exposing the Origins of the Ebola Outbreak: Urging for a Shift in Response from Reactive to Proactive. American Journal of Infectious Diseases and Microbiology, (2014); 2(6A): 1-18.
- Stahelin RV. Could the Ebola virus matrix protein VP40 be a drug target? Expert Opinion on Therapeutic Targets, (2014); 18(2): 115-120.
- Hoenen T, Biedenkopf N, Zielecki F, Jung S, Groseth A, et al. Oligomerization of Ebola virus VP40 is essential for particle morphogenesis and regulation of viral transcription. Journal of Virology, (2010); 84(14): 7053-7063.
- Kühl A, Banning C, Marzi A, Votteler J, Steffen I, et al. The Ebola virus glycoprotein and HIV-1 Vpu employ different strategies to counteract the antiviral factor tetherin. Journal of Infectious Diseases, (2011); 204(suppl 3): S850-S860.
- Chang J, Block TM, Guo J-T. Antiviral therapies targeting host ER alpha-glucosidases: current status and future directions. Antiviral Research, (2013); 99(3): 251-260.
- Ye L, Yang C. Development of vaccines for prevention of Ebola virus infection. Microbes and Infection, (2015); 17(2): 98-108.
- Huttner A, Dayer J-A, Yerly S, Combescure C, Auderset F, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. The Lancet Infectious Diseases, (2015).
- Cooper CL, Bavari S. A race for an Ebola vaccine: promises and obstacles. Trends in Microbiology, (2014); 201-2.
- Wong G, Richardson JS, Cutts T, Qiu X, Kobinger GP. Intranasal immunization with an adenovirus vaccine protects guinea pigs from Ebola virus transmission by infected animals. Antiviral Research, (2015); 11617-19.
- Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxidants & Redox Signaling, (2014); 20(11): 1810-1826.