Full Length Research Article
Toxicity, analgesic and sedative potential of crude extract of soil-borne phytopathogenic fungi Aspergillus flavus
Bashir Ahmad1, Muhammad Rizwan1*, Sadiq Azam1, Abdur Rauf2, Shumaila Bashir3
Adv. life sci., vol. 4, no. 1, pp. 14-19, November 2016
*- Corresponding Author: Muhammad Rizwan (Email: rizwanbbt55@gmail.com)
Authors' Affiliations
2- Department of Chemistry, University of Swabi, KPK – Pakistan
3- Department of Pharmacy, University of Peshawar, KPK – Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Aspergillus flavus is one of the most abundant mold present around the world. The present study was conducted to investigate the acute toxicity, analgesic and sedative effect of the crude extract obtained from soil borne fungi A. flavus.
Methods: The fungi was isolated from soil samples and identified morphologically and microscopically. The growth condition i.e. media, temperature, pH, and incubation period were optimized. In these optimized growth condition, A. flavus was grown in batch culture in shaking incubator. Crude contents were extracted by using ethyl acetate solvent. Crude secondary metabolites were screened for acute toxicity, analgesic and sedative effect.
Results: Upon completion of the experiment, blood was collected from the tail vein of albino mice, and different haematological tests were conducted. White blood cells counts displayed a slight increase (10.6× 109/L) above their normal range (0.8–6.8 × 109/L), which may be due to the increment in the number of lymphocytes or granulocytes. However, the percentage of lymphocytes was much lower (17.7%), while the percentage of the granulocytes was higher (61.4%) than its normal range (8.6–38.9%). A reduction in the mean number of writhing in the different test groups was caused by the application of the crude ethyl acetate extract through the i.p. route at different doses (50, 100, and 150 mg/kg body weight). The results of our investigation showed the EtOAc extract of A. flavus can cause a significant sedative effect in open field.
Conclusion: It was concluded from the present study that the A. flavus has the potential to produce bioactive metabolites which have analgesic and sedative effect.
Keywords: Ethyl Acetate, Aspergillus flavus, Secondary Metabolites, Haematological Analysis
Introduction
Aspergillus is one of the oldest and common genera of fungi. Aspergillus received its name due to microscopic spore-bearing structure, which resembles the Aspergillum, the instrument used to sprinkle holy water in the Roman Catholic churches [1]. Until now, more than 250 Aspergillus species have been identified [2].
Aspergillus flavus has garnered worldwide importance due to its industrial use and production of toxin. The section Flavi is categorized in two classes of species; one includes the aflatoxigenic species, e.g., A. parasiticus, A. flavus, and A. nomius, that are responsible for serious complications reported worldwide in agricultural products and the other includes the non aflatoxigenic species, e.g., A. sojae, A. tamarii, and A. oryzae. These non-aflatoxigenic species are traditionally used in the production of fermented foods in Asia [3]. A. flavus is the most important fungal species that can be found in soil and other substrates. Among the genus Aspergillus, A. flavus is the most important specie economically as well as famous due to its potential to produce aflatoxins. A. flavus, one of the most abundant soil-borne mold is a saprobe that is capable of surviving on many organic nutrient sources, plant debris, compost piles, cotton, animal fodder, dead insects, animal carcasses, outdoor and indoor environments, stored grains, and even immune compromised human and animals [4]. A. flavus is the most important economically as well as famous due to its potential to produce aflatoxins. However, not all strains of A. flavus produce aflatoxins; several strains are non-toxigenic [5]. A. flavus produces the Lovastatin (cholesterol lowering drug) [6].
Until now, very little work has been carried out on the exploration of phytopathogenic fungi. Besides, their pathogenicity, these fungi produced a wide range of beneficial compounds which can be used for the welfare of society. Therefore, realizing the need and importance of plant pathogenic fungi, the present investigation was undertaken.
Methods
Soil Sample Collection
Soil samples were collected from the vicinity of infected plants from different localities of the Malakand agency Khyber Pakhtunkhwa, Pakistan. These samples were collected in sterilized bags and were brought to the laboratory for further processing.
Isolation of Fungi
A variety of different fungal media (Potato dextrose broth, Meat extract Broth, Sabouraud dextrose broth and Czapek yeast broth) different fungi were isolated and identified microscopically and morphologically by a plant pathologist at Department of plant pathology Agricultural University Peshawar KPK, Pakistan.
Extraction of crude metabolites: Potato dextrose broth (PDB) were prepared and sterilized at 121ºC for 20 min. A five-day-old culture was inoculated in each flask containing the media [7,8]. The flasks were incubated in shaking growth condition (150 rpm) at 25ºC. After the completion of incubation period, 200 to 500 µL of 40% HCl was added to each flask which enabled the components of media to separate out. After vigorous mixing and grinding, the culture was filtered through a filter paper.
For crude metabolites, the supernatant was treated thrice with equal volume of EtOAc and concentrated with rotary evaporator under vacuum at 45ºC. The biomass and crude secondary metabolites were determined using the method of Balaraman K, Mathew (2006) with some modifications [9].
Experimental Animals
In order to perform the pharmacological tests of the crude extract, the animals were purchased form the Veterinary Research Institute (VRI), Peshawar Khyber Pakhtunkhwa, Pakistan. The guidelines prescribed by institute of laboratory animal resources, Commission on life sciences and National Research Council were strictly followed throughout the study [10]. The mice were divided into different groups; each groups consisted of six mice (n = 6). The average weight of mice was 15-22 g. The mice were kept in an air conditioned and well ventilated room, maintained at 25ºC. They were fed with chick mash pellets. Throughout the experiment, animals were also facilitated by artificial lighting provided 24 hours. The animals were kept for seven days before experiment for acclimatization.
Acute Toxicity
The crude metabolites were dissolved in propylene glycol to prepare stock solution (100 mg/mL). A dose of 5 mg/kg of body weight was injected into each mouse intraperitoneally for four consecutive days. A dose level of 10 mg/kg of body weight was administered to each group on the fifth and final day. A negative control group of mice was administered sterilized propylene glycol without the extract. During these days, the behavior of the mice was observed. The experiments were completed at the end of the seventh day. The mice were sacrificed and the blood was collected in sterilized tube, while the organs (Kidney, heart and lungs) were preserved in 10% formalin solution. In the event of mice dying in any group, the mice were subjected to post-mortem examination. The biochemical and hematological parameters (White blood cells, lymphocytes, granulocytes, red blood cells, haemoglobin, haematocrit, platelets count) were evaluated. The different organs of the sacrificed animals were weighed and gross pathology was recorded. The toxicity was graded as follow [11].
Analgesic Activity
Acetic acid induced writhing procedure was used to determine the antinociceptive/analgesic effect of crude EtOAc fraction. The mice were distributed into five groups; each group containing six mice (n = 6). Normal saline at concentration of 10 mL/kg of body weight was administered to Group I as the negative control, while diclofenac sodium at concentration of 10 mg/kg of body weight was used as a positive control (Group II). All the conditions were maintained according to the recommended guidelines. Two hours prior to the start of the experiment, the food supply was stopped [12]. The crude EtOAc extract was administered to the remaining groups, III, IV, and V at different doses, viz. 50, 100, and 150 mg/kg of body weight. After 30 min, 1% acetic acid was injected to members of all groups through the intra-peritoneal route. Abdominal writhes (constrictions) were counted after 10 min, i.e., after 5 min of acetic acid injection. The percent analgesic effect was calculated according to the following formula:
![]()
Sedative Activity
A special apparatus was used to test the sedative activity, which consisted of an area of white wood (having a diameter of 150 cm), surrounded by stainless steel. The area was divided into four squares through black lines. Before the start of the experiment, animals were adapted under red light (40 W red bulb) with water and food provided ad libitum. The animals were divided into five groups of six animals each (n = 6). Two groups, I and II were maintained as negative (normal saline) and positive control (diazepam), respectively. Normal saline (10 mL/kg body weight) was administered to Group I, while diazepam (0.5 mg/kg b.w) was administered to group II. The extracts (50, 100, 150 mg/kg b.w) were administered to the remaining groups, III, IV, and V. After 30 min, each mouse was placed in the center of the apparatus box, and the numbers of lines crossed by each mouse was counted [13].
Statistical Analysis
Results obtained were analyzed by One way ANOVA followed by Dunnett’s post hoc test.
Results
Twenty-five different soil samples were collected in sterilized polyethylene bags from different localities of Malakand District, Khyber Pakhtunkhwa, Pakistan and brought to the Microbiology Laboratory, COBAM. Using the serial dilution method and by culturing the fungi in different media, a variety of fungal strains were isolated. Among them, S. rolfsii (15 isolates), A. flavus (21), A. niger (19), and Nigrospora species (4), The isolated fungi were tested for bioactive properties. Among the bioactive isolates, two fungal species were selected for further studies.
Using optimized growth parameters, secondary metabolites were produced and isolated using EtOAc solvents. The EtOAc secondary metabolites were screened for different in vivo biological activities.
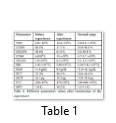
The acute toxicity of the crude EtOAc extract of A. flavus was examined in the present study. After the completion of the experiment, all animals remained alive. Thus, the crude metabolites of A. flavus also fall in the category of mild toxicity. Upon completion of the experiment, blood was collected, and different hematological tests were conducted. WBC counts displayed a slight increase (10.6× 109/L) above their normal range (0.8–6.8 × 109/L), which may be due to the increment in the number of lymphocytes or granulocytes. However, the percentage of lymphocytes was much lower (17.7%), while the percentage of the granulocytes was higher (61.4%) than its normal range (8.6–38.9%) (Table 1). The results of the haemoglobin (HGB), haematocrit (HCT) and red blood cell counts (RBC) indicated a slight decrease from their normal values, which also suggest that the extract of Aspergillus species has identical effect on RBC and WBC parameters. Overall, all findings showed that the extract does not exert an extremely toxic effect on mice.As shown in Figure 1, a reduction in the mean number of writhing in the different test groups was caused by the application of the crude EtOAc extract through the i.p. route at different doses (50, 100, and 150 mg/kg b.w).
In the group in which normal saline was administered, the mean writhing was 45.4 ±1. The percentage of writhing inhibitory effect produced by different test doses of the crude EtOAc extract was 14.097% (50 mg/kg b.w), 23.28% (100 mg/kg b.w), and 33.87% (150 mg/kg b.w). The effect generated by the crude EtOAc extract was dose dependent. At a dose of 10 mg, diclofenac sodium (positive control) caused maximum inhibition (43.10), which was more profound than the one induced by the highest dose of the crude EtOAc extract (150 mg/kg).
The results of our investigation showed the EtOAc extract of A. flavus can cause a significant sedative effect in open field. The numbers of the crossed line after 30 min are given in the Figure 2. The increase in the concentration of the crude extract (50, 100, and 150 mL or mg/kg b.w) resulted in a rise of the sedative effect (108.2 ±1.22, 91.33 ±1.83, and 81.5 ±1.83, respectively).
Tables & Figures
Discussion
A large number of new and known bioactive metabolites, such as antivirals, enzyme inhibitors, antibiotics, antihelmintics, anticarcinogens, insecticides, vitamins, antioxidants, immune-suppressants, and immunomodulatory compounds having industrial, pharmaceutical, and agricultural importance have been obtained from soil fungi [14,15]. The antimicrobial properties of secondary metabolites derived from various groups of fungi are widely reported, suggesting the outstanding potential of this microbial community as an important source of bioactive molecules [16,17].
The present study evaluated the toxic potential of crude extract obtained from selected fungi. The toxicity was categorized in different categories i.e. high, medium, moderate and mild toxicity. It was observed that the extract fall in the category of mild toxicity. Ochratoxin A and sterigmatocystin produced by Aspergillus species are examples of toxic mycotoxins. Literature reported that the common clinical signs and symptoms of toxicity caused by the extract in experimental animals were: anorexia, diarrhoea, ataxia, dyspnea, tachypnea, tachycardia, and somnolence. The effect of ataxia on the sensory and autonomic central nervous system was evaluated on the basis of their inability to control and coordinate movement. Similarly, diarrhoea, tachypnea (quick and shallow respiration), tachycardia (increased heart beat), and somnolence are signs of toxicity of the central nervous system [18].
It is suggested that the crude EtOAc extract of the A. flavus contains some bioactive compounds which exert an analgesic effect in the form of a reduction in the abdominal constriction (writhing). It was also reported that some Aspergillus species produced some bioactive secondary metabolites which have analgesic and antiulcer activities [19]. Ibuprofen is a potent analgesic compound produced by different species of fungi including Aspergillus species [20]. Any agent with sedative properties will cause a decrease in the number of movements [21]. During active cell growth, fungi produce different types of secondary metabolites (toxins, ketones, alkaloids, antibiotics, fatty acids, alcohols, etc.) [22]. These metabolites have a substantial spectrum of functions, including sedative effect [23]. This property of the metabolites of A. flavus to suppress the locomotor activity suggests that the extract possess a central nervous system (CNS) depressant activity.
It is concluded from the present study that phytopathogenic soil borne fingi A. flavus has great potential to produce a wide range of secondary metabolites which have analgesic and sedative effect, furthermore the metabolites have no toxicity. The secondary metabolites have great importance because these are renewable, eco-friendly and easily obtainable. Efforts are needed to explore these natural products to produce new drugs and other useful products.
References
- Denning DW. Invasive aspergillosis. Clinical Infectious Diseases, (1998); 26(4): 781-803.
- Geiser D, Klich M, Frisvad JC, Peterson S, Varga J, et al. The current status of species recognition and identification in Aspergillus. Studies in Mycology, (2007); 591-10.
- Kumeda Y, Asao T. Heteroduplex Panel Analysis, a Novel Method for Genetic Identification of Aspergillus Section Flavi Strains. Applied and Environmental Microbiology, (2001); 67(9): 4084-4090.
- Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Allameh A, Kazeroon-Shiri A, Ranjbar-Bahadori S, et al. A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia, (2006); 161(3): 183-192.
- Ordaz JJ, Fente C, Vázquez B, Franco C, Cepeda A. Development of a method for direct visual determination of aflatoxin production by colonies of the Aspergillus flavus group. International Journal of Food Microbiology, (2003); 83(2): 219-225.
- Nidhiya K, Sathya E, Nitya M. Extraction and purification of lovastatin from non-aflatoxigenic strains of Aspergillus flavus. International Journal of Biological & Pharmacutical Research, (2012); 4916-921.
- Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nature Protocols, (2010); 5(3): 479-490.
- Khattak SU, Iqbal Z, Lutfullah G, Bacha N, Khan AA, et al. Phytotoxic and herbicidal activities of Aspergillus and Penicillium species isolated from rhizosphere and soil. Pakistan Journal of Weed Science Research, (2014); 20(3): 293-303.
- Balaraman K, Mathew N. Optimization of media composition for the production of cyclosporin A by Tolypocladium species. Indian Journal of Medical Research, (2006); 123(4): 525.
- Khan H, Saeed M, Khan MA, Dar A, Khan I. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. Journal of Ethnopharmacology, (2010); 127(2): 521-527.
- Pathak B, Sethi N, Gupta J, Vora V. Toxicity studies of metabolites of some fungal isolates in albino mice. Applied and Environmental Microbiology, (1983); 46(4): 944-947.
- Muhammad N, Saeed M, Khan H, Haq I. Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. Journal of Natural Medicines, (2013); 67(1): 1-8.
- Han BH, Park MH. Sedative activity and its active components of Zizyphi fructus. Archives of Pharmacal Research, (1987); 10(4): 208-211.
- Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiology and Molecular Biology Reviews, (2003); 67(4): 491-502.
- Makut M, Owolewa O. Antibiotic-producing fungi present in the soil environment of Keffi metropolis, Nasarawa state, Nigeria. Eubacteria, (2011); 10(18): 19.
- Sekiguchi J, Gaucher GM. Conidiogenesis and secondary metabolism in Penicillium urticae. Applied and Environmental Microbiology, (1977); 33(1): 147-158.
- Schulz B, Boyle C, Draeger S, Römmert AK, Krohn K. Endophytic fungi: a source of novel biologically active secondary metabolites. Mycological Research, (2002); 106(09): 996-1004.
- Cole RJ, Kirksey JW, Dorner JW, Wilson DM, Johnson Jr JC, et al. Mycotoxins produced by Aspergillus fumigatus species isolated from molded silage. Journal of Agricultural and Food Chemistry, (1977); 25(4): 826-830.
- Pinheiro EAA, Carvalho JM, dos Santos DCP, Feitosa AdO, Marinho PSB, et al. Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Natural Product Research, (2013); 27(18): 1633-1638.
- Chen CS, Shieh WR, Lu PH, Harriman S, Chen CY. Metabolic stereoisomeric inversion of ibuprofen in mammals. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, (1991); 1078(3): 411-417.
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology, (2003); 463(1): 3-33.
- Devi P, D'Souza L, Kamat T, Rodrigues C, Naik CG. Batch culture fermentation of Penicillium chrysogenum and a report on the isolation, purification, identification and antibiotic activity of citrinin. Indian Journal of Marine Sciences, (2009); 38(1): 38.
- Bachelor F, King G. Chemical constituents of lichens: aphthosin, a homologue of peltigerin. Phytochemistry, (1970); 9(12): 2587-2589.