Full Length Research Article
The effect of N-acetyl cysteine on H2O2 mediated oxidative stress in Whartonʼs jelly derived mesenchymal stem cells
Fatima Ali*, Abdu Ur Rehman Qadir, Nishat Fatima, Nadia Wajid
Adv. life sci., vol. 4, no. 4, pp. 137-142, August 2017
*- Corresponding Author: Fatima Ali (Email: fatemei.ali@gmail.com)
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Hypoxic stress is a crucial factor for retaining the cell survival in injured tissue. Overcoming this issue is the key for successful cellular regenerative therapy. Therefore the purpose of this study was to investigate whether the in-vitro pretreatment of Whartonʼs Jelly (WJ) derived Mesenchymal stem cells (WJ-MSCs) with an antioxidant, namely N-acetylcysteine (NAC), can improve the efficacy of WJ-MSCs for transplantation purpose.
Methods: WJ-MSCs were cultured with or without NAC at different concentrations (0.1mM, 1mM and 10mM). To simulate oxidative stress conditions, cultures were exposed to hydrogen peroxide (H2O2) 100 µM for 1 hour. Cytoprotective effect of NAC was evaluated by determining cell injury, viability, and proliferation. The oxidative stress is assessed by measuring the activity of glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and malodialdehyde (MDA).
Results: Pretreatment of WJ-MSCs with NAC increased their viability and proliferation in concentration-dependent manner. Furthermore, 10 mM NAC significantly reduced the H2O2 induced oxidative stress by enhancing the activity of GSH, SOD, and CAT and reduced the level of MDA
Conclusion: The study results indicate that NAC may abrogate H2O2 induced oxidative-stress of WJ-MSCs. This study provides basis to explore NAC effect on WJ-MSCs survival without cytotoxicity.
Key words: Whartonʼs Jelly, N-acetylcysteine, hydrogen peroxide, oxidative stress
Introduction
Wharton’s jelly derived mesenchymal stem cells (WJ-MSCs) have great potential in regenerative medicine and cellular therapy [1]. Cell therapy is based on transplantation of stem cells to repair damaged tissue but the transplanted cells face hypoxic environment in the tissue which may lead to apoptosis of cells [2,3]. To overcome this situation, in vitro preconditioning of stem cells is well established strategy [2,4-6]. In this study WJ-MSCs are in vitro preconditioned with N-acetylcysteine (NAC). NAC is a cysteine analogue drug used as a glutathione (GSH) precursor [4]. GSH acts as an antioxidant to reduce the oxidative stress. Recent studies showed that the oxidative stress is increased in injury condition due to overproduction of reactive oxygen species (ROS) [7].
ROS include hydrogen peroxide (H2O2), hydroxyl radicals (OH-), and superoxide (O2−), these may cause deleterious effects on living systems [8]. The damaging effect of ROS termed as oxidative stress decreases the efficiency of both enzymatic and non-enzymatic antioxidants. Recent studies have underlined the protective effect of NAC by reducing the ROS and preventing the damage to many organs [9-11].
It is hypothesized that pretreatment of WJMSCs with NAC could be a promising therapeutic approach to improve cell survival. Therefore, the purpose of this study was to investigate the role of pretreatment of WJ-MSCs with NAC to improve the efficacy of WJ-MSCs.
Methods
Procurement of human umbilical cord
Umbilical cords were obtained after full term birth (cesarean section) with the informed consent of the parents using the guidelines approved by the Biosafety Board at The University of Lahore, Pakistan. Donors were tested for Hepatitis B and C virus (HBV and HCV) and only HBV and HCV negative donors were selected. Umbilical cord tissue was stored in sterile normal saline (0.9% w/v sodium chloride), until processing.
WJ-MSCs cell culture
Isolation of MSCs from WJ was done by explant culturing method (Sigma Aldrich, USA) as previously reported [12]. Briefly, the cord sections were incubated in 3 mg/mL collagenase solution (Sigma Aldrich, USA). After 3 hours of incubation, Dulbecco's modified eagle medium low glucose (DMEM LG; Sigma Aldrich) with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NJ) and 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Grand Island, N.J., USA) was added. The medium was renewed after every 3 days. The cells of passage 3 were used in the study.
Experimental design
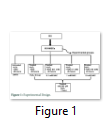
WJ-MSCs were randomly divided into five experimental groups: untreated with both H2O2 and NAC (Untreated; UNTR), 100 µM H2O2 treated control group (H2O2 control group; H2O2); WJ-MSCs treated with 0.1mM concentration of NAC (Sigma Aldrich, USA) (0.1mM NAC group); with 1mM concentration of NAC (1mM NAC group); and 10 mM concentration of NAC (10mM NAC group) for 48 hours [16] and subsequently treated with 100 µM H2O2 for 1 hour (NAC group). 100 µM H2O2 dose was selected in this investigation based on a series of preliminary test experiments. All experiments were performed in serum free DMEM for 48 hours (Figure 1).
Cell viability assay
After treatment cells were washed with PBS and then stained with crystal violet (Sigma Aldrich., USA) for 20 minutes which were subsequently lysed with SDS after three washings with PBS. Absorbance was taken at 595 nm.
Cell proliferation assay
To compare the proliferative potential of experimental groups, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was performed. Monolayer of cells was first washed with phosphate buffer saline (PBS) (Invitrogen Inc., USA). 500 µL complete medium along with 60 µL MTT solution (Invitrogen Inc., USA) was added to cells and incubated for 2 hours at 37°C. Purple color crystals formed within cells were solubilized with DMSO and absorbance was taken at 570 nm.
LDH assay
LDH assay was performed using 5µL medium from each group at the end of treatment using LDH assay kit (AMP Diagnostics, Austria) according to manufacturer’s instructions. Briefly, cell culture medium (5 µL) of both groups was mixed with working reagent (95 µL), incubated for 5 minutes and then absorbance was recorded at wavelength of 340 nm.
Determination of oxidative stress
The oxidative stress markers, including activities of glutathione reductase (GSH), catalase (CAT), and superoxide dismutase (SOD), and level of Malondialdehyde (MDA) were determined, according to procedure previously described [13].
Determination of SOD activity
Homogenate was prepared by mixing serum and trichloroacetic acid (50%) in 1:1 ratio and centrifuged at 13,000 rpm for 10 minutes at 25°C. 15 µL supernatant was added to 120 µL sodium pyrophosphate buffer (52 mM, pH 8.3), 12 µL phenazine methosulphate, 36 µL nitroblue tetrazolium. Reaction was started by addition of 24 µL nicotinamide adenine dinucleotide. After incubation at 37°C for 90 seconds, reaction was stopped by addition of 12 µL of glacial acetic acid. The reaction mixture was stirred vigorously with 400 µL of n-butanol. The mixture was incubated for 10 minutes and then centrifuged at 2000 rpm for 5 minutes at 25°C and butanol layer was separated. The color intensity of chromogen in n-butanol layer was measured at 560 nm against n-butanol using a spectrophotometer.
Estimation of reduced GSH
Homogenate was prepared by mixing serum and trichloroacetic acid (10%) in 1:1 ratio and centrifuged at 1000 rpm for 10 minutes at 25°C. 40 µL supernatant was mixed with 150 µL of 0.3 M disodium phosphate buffer. Then 25 µL of 0.001 M freshly prepared DTNB [5,50-dithiobis (2-nitrobenzoic acid) dissolved in 1% sodium citrate] was added. Reduction of DTNB with GSH produced a yellow compound, whose absorbance was noted spectrophotometrically at 412 nm. The reduced chromogen is directly proportional to GSH concentration.
Estimation of catalase activity
Serum (40 µL) was mixed with 360 µL phosphate buffer (10 mM, pH 7.0) and centrifuged at 13,000 rpm for 10 minutes at 25°C. 21 µL of the supernatant and 180 µL phosphate buffer (10 mM, pH 7.4) were mixed. Reaction was started by addition of freshly prepared 75 µL H2O2 (0.2 M). 360 µL potassium dichromate acetic acid reagent (5%) was added to reaction mixture and incubated for 10 minutes in boiling water, cooled, and absorbance was measured at 530 nm.
Estimation of MDA level
For this, 40 µL of serum was taken and a homogenate was prepared in 360 µL phosphate buffers (10 mM, pH 7.4) and centrifuged at 13,000 rpm for 10 minutes at 25°C. 15 µL supernatant was mixed with 15 µL SDS (8.1%), 96 µL TBA (0.8%), 96 µL acetic acid (20%) and 18 µL distilled water and incubated at 90°C for 1 hour. Afterwards, 60 µL distilled water and 300 µL n-butanol-pyridine mixture (15:1) was added and the mixture was shaken vigorously and centrifuged at 4000 rpm at 25°C for 10 minutes. The upper n-butanol layer was separated and its absorbance was taken at 532 nm.
Statistical analysis
The experimental data were expressed as the mean ± standard error of mean (SEM). Statistical analysis was performed using GraphPad Prism 5 software (GraphPad, San Diego, CA). The mean data of all groups were compared with one-way ANOVA, followed by Scheffe’s post hoc test for multiple comparisons. The P<0.05 value was considered as statistically significant.
Results
Cytoprotective effect of NAC on WJ-MSCs
Cells’ viability
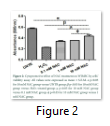
The crystal violet assay was performed to check the cells’ viability. Preconditioning with 0.1 mM and 1 mM NAC concentrations resulted in increased cell viability (0.34 ± 0.010 nm; 0.35 ± 0.002 nm) but not more than 10 mM NAC group (0.44 ± 0.018 nm). Figure 2 shows that cell viability level is significantly lower in H2O2 group (0.23 ± 0.004 nm) compared with 10 mM NAC group.
Cells’ proliferation
Cells’ proliferation potential of treatment groups was assessed by performing MTT assay. Preconditioning with 0.1 mM and 1 mM NAC concentrations resulted in increased cell proliferation (0.49 ± 0.002; 0.55 ± 0.000) but 10 mM NAC group (0.62 ± 0.000) showed even better results. Figure 3 shows that proliferation is significantly low in H2O2 group (0.40 ± 0.009) compared with 10 mM NAC group.
Lactate dehydrogenase (LDH) release
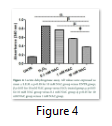
The membrane integrity of cells was determined by performing LDH cytotoxicity assay. As shown in Figure 4, LDH release was significantly decreased in 0.1 mM and 1 mM NAC groups (0.77 ± 0.001; 0.57 ± 0.008) while the best results were observed with 10 mM NAC group (0.38 ± 0.004). Figure 4 shows that LDH release was significantly high in H2O2 group (0.86 ± 0.000) compared with 10mM NAC group.
Oxidative stress
Antioxidant enzymes status was evaluated by spectrophotometric assays (Figure 5). Figure 5A showed that pre-incubation of cells with 0.1 mM and 1 mM NAC concentraion resulted in increased SOD level (0.06 ± 0.000; 0.07 ± 0.001) but not more than 10 mM NAC group (0.12 ± 0.001). SOD level is significantly less in H2O2 group (0.05 ± 0.004) compared with10 mM NAC group.
Figure 5B showed that preconditioning of cells with 0.1 mM and 1 mM NAC concentration resulted in increased GSH level (0.96 ± 0.018; 1.19 ± 0.048) which is even better with 10 mM NAC group (1.33 ± 0.025). GSH level is significantly decreased in H2O2 group (0.59 ± 0.000) compare with10 mM NAC group.
Figure 5C showed that preconditioning of cells with 0.1 mM and 1 mM NAC concentration which resulted in an increased CAT activity (0.58 ± 0.004; 0.44 ± 0.000) which is significantly increased in 10 mM NAC group (0.29 ± 0.004). CAT activity is significantly decreased in H2O2 group (0.64 ± 0.010) compared with10 mM NAC group.
Figure 5D showed that preconditioning of cells with 0.1 mM and 1 mM NAC concentration resulted in increased MDA level (0.31 ± 0.077; 0.29 ± 0.002) which is better with 10 mM NAC group (1.79 ± 0.002). MDA level is significantly increased in H2O2 group (0.59 ± 0.002) compared with10 mM NAC group.
Tables & Figures
Discussion
Oxidative stress acts as a major contributor to morbidity and mortality in patients as it hampers the repair potential of stem cells [14,15]. The functional capability of stem cells to regenerate injured tissue is critical to repair the organ. WJ-MSCs are pluripotent therefore; they are used in cell based therapies [5,16,17]. Hypoxic stress affects the MSCs’ proliferation and function which warrants a strategy to increase functioning of affected MSCs for stem cell-based therapies [18]. Thus, different strategies are required which can augment the functioning of stem cells in hypoxic microenvironment [6,19]. Preconditioning of MSCs with antioxidant is one of the potential strategies to address this issue [20]. So, we devised a strategy to improve the behavior of WJMSCs.
Therefore, the aim of present study was to devise a preconditioning strategy to improve the cell survival of WJMSCs in hypoxic condition. In this study the effect of NAC on the behavior of WJMSCs was evaluated. In the current study, H2O2 stimulation resulted in reduced cell viability (Figure 2), cell proliferation (Figure 3), and increased LDH release (Figure 4) in accordance with previous studies [21,22]. NAC resulted in enhanced cell viability, cell proliferation and reduced LDH release and it conferred protection against H2O2-induced cellular injury.
NAC preconditioned WJMSCs were subjected to hypoxic injury to mimic the hypoxic tissue microenvironment. In injury condition ROS production elicit the disease progression due to reaction with DNA, protein and lipids all these events are reported in many cases [23,24]. It has been previously shown that the antioxidant activity is dependent on ROS level [6,25]. Previous studies have shown that ROS affect the antioxidant defense mechanisms by decreasing the activity of SOD and CAT [26], and also reducing the intracellular concentration of GSH [27]. As the level of hypoxic stress increases, it decreases the antioxidant level, this may explain the observed low antioxidant level (Figure 5A-D). The study results showed that low antioxidant enzyme level was significantly improved after NAC treatment as evidenced in Figure 5A-C. Pirinccioglu et al. have demonstrated that ROS cause peroxidation of polyunsaturated lipids and produces polyunsaturated precursors such as MDA, which is used as an oxidative stress biomarker [28]. Figure 5D coincides with similar findings as it demonstrates high level of lipid peroxidation product MDA in hypoxic group.
Acknowledgment
This work was supported by research grants from The University of Lahore, Lahore, Pakistan.
The authors declare no conflict of interest.
References
- Kim D-W, Staples M, Shinozuka K, Pantcheva P, Kang S-D, et al. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. International Journal of Molecular Sciences, (2013); 14(6): 11692-11712.
- Khan M, Ali F, Mohsin S, Akhtar S, Mehmood A, et al. Preconditioning diabetic mesenchymal stem cells with myogenic medium increases their ability to repair diabetic heart. Stem Cell Research & Therapy, (2013); 4(3): 58.
- Saini U, Gumina RJ, Wolfe B, Kuppusamy ML, Kuppusamy P, et al. Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation. Journal of Cellular Biochemistry, (2013); 114(11): 2612-2623.
- Ali F, Khan M, Khan SN, Riazuddin S. N-Acetyl cysteine protects diabetic mouse derived mesenchymal stem cells from hydrogen-peroxide-induced injury: A novel hypothesis for autologous stem cell transplantation. Journal of the Chinese Medical Association, (2016); 79(3): 122-129.
- Ali F, Wajid N. N-acetylcysteine prevents cord derived stem cells from H2O2 induced injury in vitro. European Journal of Pharmaceutical and Medical Research, (2015); 2(3): 589-598.
- Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Translational Stroke Research, (2013); 4(1): 76-88.
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation Research, (2010); 107(9): 1058-1070.
- Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxidative Medicine and Cellular Longevity, (2013); 2013956792.
- Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N‐acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain and Behavior, (2014); 4(2): 108-122.
- Xie J, Zhou X, Hu X, Jiang H. H2O2 evokes injury of cardiomyocytes through upregulating HMGB1. Hellenic Journal of Cardiology, (2014); 55(2): 101-106.
- Sun Y, Pu LY, Lu L, Wang XH, Zhang F, et al. N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World Journal of Gastroenterology, (2014); 20(41): 15289.
- Wajid N, Naseem R, Anwar SS, Awan SJ, Ali M, et al. The effect of gestational diabetes on proliferation capacity and viability of human umbilical cord-derived stromal cells. Cell and Tissue Banking, (2015); 16(3): 389-397.
- Ali F, Naqvi SAS, Bismillah M, Wajid N. Comparative analysis of biochemical parameters in diabetic and non-diabetic acute myocardial infarction patients. Indian Heart Journal, (2016); 68(3): 325-331.
- Sung CC, Hsu YC, Chen CC, Lin YF, Wu CC. Oxidative stress and nucleic acid oxidation in patients with chronic kidney disease. Oxidative Medicine and Cellular Longevity, (2013); 2013301982.
- Ansley DM, Wang B. Oxidative stress and myocardial injury in the diabetic heart. The Journal of Pathology, (2013); 229(2): 232-241.
- Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, et al. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. BioMed Research International, (2013); 2013916136.
- Barachini S, Trombi L, Danti S, D’Alessandro D, Battolla B, et al. Morpho-functional characterization of human mesenchymal stem cells from umbilical cord blood for potential uses in regenerative medicine. Stem Cells and Development, (2009); 18(2): 293-306.
- Hung S-C. Effects of hypoxic culture on bone marrow mesenchymal stem cells: from bench to bedside. Formosan Journal of Surgery, (2013); 46(2): 35-38.
- Haider HK, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. Journal of Molecular and Cellular Cardiology, (2008); 45(4): 554-566.
- Li CJ, Sun LY, Pang CY. Synergistic protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Scientific Reports, (2015); 5: 59819.
- Li P, Li Z. Neuroprotective effect of paeoniflorin on H2O2-induced apoptosis in PC12 cells by modulation of reactive oxygen species and the inflammatory response. Experimental and Therapeutic Medicine, (2015); 9(5): 1768-1772.
- Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, et al. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2, 3, 4-trimethoxybenzyl] piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. Journal of Pharmacology and Experimental Therapeutics, (2009); 329(2): 543-550.
- Frank J, Pompella A, Biesalski H. Histochemical visualization of oxidant stress. Free Radical Biology and Medicine, (2000); 29(11): 1096-1105.
- Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. Journal of Toxicology, (2012); 2012645460.
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clinical Interventions in Aging, (2007); 2(2): 219.
- Li J-L, Wang Q-Y, Luan H-Y, Kang Z-C, Wang C-B. Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. Journal of Biomedical Science, (2012); 19(1): 32.
- Majumdar D, Bhonde R, Datta I. Influence of ischemic microenvironment on human Wharton's Jelly mesenchymal stromal cells. Placenta, (2013); 34(8): 642-649.
- Pirinccioglu AG, Gökalp D, Pirinccioglu M, Kizil G, Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clinical Biochemistry, (2010); 43(15): 1220-1224.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0/