Full Length Research Article
An In-Silico Approach for the Prediction of miRNAs in Merkel Cell Polyoma Virus and its Target Genes
Gohar Rahman, Bilal Ahmad Mian, Najib Ullah, Huzaifa Khan, Shaker Khan
Adv. life sci., vol. 6, no. 1, pp. 41-47, November 2018
*- Corresponding Author: Gohar Rahman (Email: grkhan117@gmail.com)
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: For last five years, as technological advancement occurs, novel kinds of human generation viruses’ discovery have been increased. A rare human cancer associated virus Merkel’s Cell polyomavirus (MCPyV or MCV) got remarkable attraction among the newly discovered viruses. As a common human virus MCPyV infection frequently found in skin and also occurs at other anatomical sites.
Methods: In this study, the in-silico screening of miRNAs from MCPyV was done, as the computational screening procedures are functionally vital, efficient and inexpensive for the said purpose.
Results: Primarily 52 sequences, possessing possible hairpin-like structures, were extracted from MCPyV genome by searching through VMir software using various filters. 17 nominees were confirmed with real pre-miRNA like hairpin organizations by iMiRNA-SSF program. Further seven nominees were excluded by free energy measurement and other parameters. 10 mature miRNAs were affirmed in 10 impending candidates for pre-miRNAs by MatureBayes web server v1.0, among these 10 candidates 2 were reported already by earlier studies. The homologous miRNA for these candidates were searched in mirBase. To find the target gene and its relevant diseases the best homologous miRNAs were then searched in Target Scan. Among all miRNAs Cancer was found to be common.
Conclusion: These findings open new avenues for researchers to explore the role of these novel miRNAs in viral pathogenesis as well as in developing new antiviral therapy.
Keywords: Merkel Cell Carcinoma, Merkel Cell Polyomavirus. miRNAs, Computational Tools
Merkel Cell Polyomavirus (MCPyV) were first identified in 2008 in the tissue from Merkel Cell Carcinoma (MCC) [1]. Natural host of polyomavirus is human and it is the initiating origin of tumor in its host. The virus is reportedly linked with MCC pathogenesis as evident from the monoclonal sequences with evident mutations and expression of T antigens in tumor tissue [2]. Epidemiological studies suggest that most of the adult healthy population is infected with the MCPyV infection during their premature age [3-7]. Therefore, MCPyV infection consequently has a rare incidence of MCC [1-8]. Large and small T antigens (LT and sT-Ag) and structural antigens VP1 and VP2 are coded by MCPyV as of all polyomaviruses. While VP3 encoding by MCPyV is still unknown to us [9]. Polyomavirus T antigens formation occurs due to earlier transcription of an alternatively spliced gene transcript during infection. Thus in MCPyV, a 57K T antigen is formed as a result of alternative splicing of initial transcript [8]. The presence of an alternative open reading frame (ALTO) has been unleashed during recent study, it could be purposely built structure created by T-Ag translational transcripts. The role of ALTO is still unclear though it shows likenesses, with the middle T antigen (mT-Ag) of some other polyomaviruses, in some sequence features. Experimentations unveil that viral DNA replication has not been effected due to existence or nonexistence of ALTO [10]. In addition to the protein products, the MCPyV has been reported with a single encoded microRNA (miRNA) originator which transcribes a couple of mature miRNAs (mcv-miR-M1-3p and mcv-miR-M1-5p) [11]. miRNA is a small fragment of non-coding RNAs (with ~22 nt.) made in result of nucleases Dicer and Drosha sequence processing action on primarily arranged transcripts [12]. After the incorporation of miRNA into RNA-induced silencing complex (RISC), the transcript expression can be destructively regulated by mature miRNA which is identified through complementary sequences. The supposed seed sequence (nt 2-8) pairing of miRNA by flawless Watson – Crick Model, the target site is identified, while in animals sequence complementarity is reduced due to differences in sequences [13]. Partial mRNA pairing can inhibit translational activities of mRNA as a result we can see a minor decrease in translation activity although. In comparison to the rare miRNA activities of animal, plant miRNA affected by animal viruses can also form bonding with target sequences with high precision of complementarity and result into endonucleolytic cleavage of mRNA mediated by RISC.
Latest studies suggest the miRNA encoding by several animal and human polyomaviruses [11,14-19]. Although detected with in various genomic loci, all of the discovered polyomavirus miRNA are sequence encoded, the same sequences are found to be of antisense orientation to the primary expressing transcript of T antigen. Therefore, flawless complementarity to initial transcript is to be found in mature miRNA species encoded by these loci, while a number of studies demonstrated that all known polyomaviruses are able to regulate translational activity of early gene product negatively [11,15,17-20]. During last decade, various techniques like cloning of cDNA and its confirmation using Northern blotting have been used in common for determination of miRNA in different organisms, including viruses also [21-24]. Still the procedures are cost-effective, time consuming and stressful [25]. While some other techniques with more efficiency, convenience and lower cost, such as computer based prediction strategies for novel miRNAs, are available for this purpose.
Sequence Retrieval
FASTA format of Merkel Cell Polyomavirus Sequence (accession no NC_010277.2) were retrieved from NCBI http://www.ncbi.nlm.nih.gov. The genome size of Merkel Cell Polyoma virus is about 5387.
Pre-miRNA Extraction
The identification of pre-miRNA candidates was performed by specialized ab initio viral pre-miRNAs prediction software VMir on the basis of comparison to structural characteristics of already recognized pre-miRNA hairpins. In order to extract hairpin-structured miRNA precursors, the viral genome was scanned by VMir software (program version 2.3, scoring algorithm version 1.4) [26,27]. The stringency were kept 10% with minimum score of 80. Primarily, potential hairpin shaped sequences were mined as candidate miRNA precursors (pre-miRNAs).
Real pre-miRNA Confirmation
For filtering real pre-miRNA from pseudo pre-miRNA, iMiRNA-SSF web server was used [28].
Identification of Unique and Potential pre-miRNA Structures
RNAfold web server (http://rna.tbi.univie.ac.at//cgi-bin/RNAWebSuite/RNAfold.cgi) was used to predict secondary structure and minimum free energy (MFE). Sequences with hairpin-like secondary structures, and having lower MFE (equal to or less than –25 kcal/mol) acts as potential miRNA precursors. Sequences having hairpin-like structures and lower MFE were selected and were confirmed to be unique by conducting BLASTn searches.
Mature miRNA Prediction
Prediction of mature miRNAs in MCpV was performed on MatureBayes web server v1.0 (http://mirna.imbb.forth.gr/MatureBayes.html). This computer-based web tool uses a Naive Bayes classifier which is based on secondary structure and sequence features of the pre-miRNAs for the prediction of mature miRNAs in any given pre-miRNA [29]. MatureBayes compute the probabilistic start position of mature miRNA(s) by two alternatives.
Targets of the miRNAs
The mature miRNAs were searched in miRbase [30]. For finding its similar miRNA. The similar miRNA found in miRBase were selected for finding target via Target Scan [31].
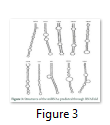
VMir is a user friendly software especially designed for viral miRNAs identification [26]. By using RNAfold algorithm, VMir executes structure prediction by minimal folding free energy and detects individual hairpins above a certain size limit (by default 45 nt). VMir assigns score to these hairpins which is based on statistical comparison to a reference set of recognized pre-miRNA hairpins [27]. In this study, the MCPyV genome uploaded to VMir Analyzer was in FASTA format. The parameters for the program was set to window size 1000 and allowed to operate within the parameters and step size was adjusted. To extract hairpin like sequences (pre-miRNAs) the viral genomes were scanned in both orientations. Initially 187 sequences were detected by VMir Analyzer as candidate miRNA precursors (Figure 1). A filter was set to pass these 187 pre-miRNA candidates through it. Only 52 hairpins passed the filter. (Figure 2). Figures 1 and 2 show the location and VMir scores for unfiltered and filtered hairpins, respectively. The real and pseudo pre-miRNAs were differentiated through iMiRNA-SSF online web server by providing the selected 52 pre-miRNA nominees [28]. Among 52 filtered sequences, 17 sequences were confirmed real pre-miRNA with fold-back hairpin shaped structures by IMiRNA-SSF. The number of real pre-miRNA decreased to 10 as lower minimum free energy was measured (equal to or less than –25 kcal/mol) and BLASTn was done to find its unique nature. RNAfold program was used to accomplish the confirmation of potential hairpin-like secondary structures in these sequences (Figure 3). Mature miRNA sequences inside pre-miRNA hairpin was identified by MatureBayes web server [29]. 10 mature miRNA within the 10 duplexes were predicted by this computational tool (Table 1). To find the similar sequences in human these 10 mature miRNAs were screened in mirBAse. The homologous miRNA sequences were selected for their target recognition. The genes targeted by miRNAs, were found by scanning the sequences in Target scan. The cancer causing top target gene are shown in table 2.
Tables & Figures
In this study, the MCPyV genome was analyzed through several bioinformatics tools. Resulting into the recognition of 10 mature miRNAs. Among these 10 mature mi-RNAs, 2 were reported before and the target genes for these sequences were identified regarding their similarity bases with other miRNAs.
Among the other different causes of cancer, viruses are being reported as the most vital source of cancer development. According to the reports of International Agency for Research on Cancer, up to 20% of cancer cases around the world are due to the infection of viruses [32]. Currently, 10%-15% human cancers are caused by seven human oncogenic viruses. Merkel cell carcinoma is a rare and highly malignant primary neuroendocrine carcinoma of the skin. Over 90% of cases arise in sun-exposed areas, with half around the cancer near to areas of head and neck. This indicates, in development of MCC, sunlight specifically the ultraviolet radiation play a key role. MCC can also be observed on the torso (trunk of the human body) and genitals with more reduced frequency (<10%) [33]. Numerous polyomaviruses, including John Cunningham Virus (JCV), simian virus 40 (SV40) and BK Virus (BKV) encodes miRNA and control viral transcript level at earlier stage [17]. Therefore, it is predictable that, In addition to the structural proteins, a 22 nucleotide long miRNA, MCV-miR-M1-5p are also encoded by late region [11]. The miRNAs are encoded antisense to the LT coding region. Complementary to a segment of the LT transcript, it regulates the early gene expression by limiting the early gene transcript. In at least half of the MCPyV-positive MCC tumors its expression is conserved, and it can play role in cellular remodeling [34].
In this study, a number of methodologies were applied for the prediction of miRNAs that were previously unidentified. The MCV genome was taken and run on VMir. The resulted 52 pre-miRNAs were obtained after the filters were applied. Pseudo miRNA was eliminated and real pre-miRNA was selected by using iMiRNA-SSF web server. The possible potential miRNA precursors were predicted using RNAfold. For further analysis miRNAs with less than -25 kcal/mol energy were selected. For mature miRNA prediction, MatureBayes web server was used. In previous study on HBV genome, 12 mature miRNAs were predicted in 6 potential pre-miRNA candidates [35]. Through target scan the mature miRNAs were searched for homologous miRNAs in miRBase, the target of the related miRNAs found in miRBase were searched. In most of the patients’ current therapies for MCV infection does not work. For novel antiviral therapeutic strategies improved information on MCV-host interaction is compulsory. The biological significance of miRNA can be defined by the relationship of viral encoded miRNAs and pathogenic characteristics of virus, this signifies the role of miRNA in therapeutic activities against a great number of diseases and can triggered a whole new era of treatment with high influence and good mechanism. In silico prediction of miRNAs is just the beginning of miRNA study and should be employed by other investigations such as target and function determination for an extensive understanding of its biological significance.
This study provides an insight to the prediction of miRNAs of MCV and its similar miRNAs in Humans and its target by using several bioinformatics tools.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science, (2008); 319(5866): 1096-1100.
- Chang Y, Moore PS. Merkel cell carcinoma: a virus-induced human cancer. (2012).
- Chen T, Hedman L, Mattila PS, Jartti T, Ruuskanen O, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. Journal of Clinical Virology, (2011); 50(2): 125-129.
- Pastrana DV, Wieland U, Silling S, Buck CB, Pfister H. Positive correlation between Merkel cell polyomavirus viral load and capsid-specific antibody titer. Medical microbiology and immunology, (2012); 201(1): 17-23.
- Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell host & microbe, (2010); 7(6): 509-515.
- Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. International journal of cancer, (2009); 125(6): 1250-1256.
- Zhang C, Liu F, He Z, Deng Q, Pan Y, et al. Seroprevalence of Merkel cell polyomavirus in the general rural population of Anyang, China. PLoS One, (2014); 9(9): e106430.
- Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proceedings of the National Academy of Sciences, (2008); 105(42): 16272-16277.
- Schowalter RM, Buck CB. The Merkel cell polyomavirus minor capsid protein. PLoS pathogens, (2013); 9(8): e1003558.
- Carter JJ, Daugherty MD, Qi X, Bheda-Malge A, Wipf GC, et al. Identification of an overprinting gene in Merkel cell polyomavirus provides evolutionary insight into the birth of viral genes. Proceedings of the National Academy of Sciences, (2013); 110(31): 12744-12749.
- Seo GJ, Chen CJ, Sullivan CS. Merkel cell polyomavirus encodes a microRNA with the ability to autoregulate viral gene expression. Virology, (2009); 383(2): 183-187.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, (2004); 116(2): 281-297.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell, (2009); 136(2): 215-233.
- Cantalupo P, Doering A, Sullivan CS, Pal A, Peden K, et al. Complete nucleotide sequence of polyomavirus SA12. Journal of virology, (2005); 79(20): 13094-13104.
- Chen CJ, Cox JE, Azarm KD, Wylie KN, Woolard KD, et al. Identification of a polyomavirus microRNA highly expressed in tumors. Virology, (2015); 476: 43-53.
- Chen CJ, Cox JE, Kincaid RP, Martinez A, Sullivan CS. Divergent microRNA Targetomes of Closely-related Circulating Strains of a Polyomavirus. Journal of virology, (2013); 01713.
- Seo G, Fink L, O'Hara B, Atwood W, Sullivan C. Evolutionarily conserved function of a viral microRNA. Journal of virology, (2008); 82(20): 9823-9828.
- Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature, (2005); 435(7042): 682.
- Sullivan CS, Sung CK, Pack CD, Grundhoff A, Lukacher AE, et al. Murine Polyomavirus encodes a microRNA that cleaves early RNA transcripts but is not essential for experimental infection. Virology, (2009); 387(1): 157-167.
- Broekema NM, Imperiale MJ. miRNA regulation of BK polyomavirus replication during early infection. Proceedings of the National Academy of Sciences, (2013); 201301907.
- Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, et al. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proceedings of the National Academy of Sciences, (2005); 102(15): 5570-5575.
- Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, et al. Identification of microRNAs of the herpesvirus family. Nature methods, (2005); 2(4): 269.
- Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, et al. Identification of virus-encoded microRNAs. Science, (2004); 304(5671): 734-736.
- Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. Journal of virology, (2005); 79(14): 9301-9305.
- Cui C, Griffiths A, Li G, Silva LM, Kramer MF, et al. Prediction and identification of herpes simplex virus 1-encoded microRNAs. Journal of virology, (2006); 80(11): 5499-5508.
- Sullivan CS, Grundhoff A. Identification of viral microRNAs. Methods in enzymology, (2007); 427: 3-23.
- Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. Rna, (2006); 12(5): 733-750.
- Chen J, Wang X, Liu B. IMiRNA-SSF: improving the identification of MicroRNA precursors by combining negative sets with different distributions. Scientific reports, (2016); 619062.
- Gkirtzou K, Tsamardinos I, Tsakalides P, Poirazi P. MatureBayes: a probabilistic algorithm for identifying the mature miRNA within novel precursors. PloS one, (2010); 5(8): e11843.
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic acids research, (2007); 36(suppl_1): D154-D158.
- Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. elife, (2015); 4e05005.
- Parkin DM. The global health burden of infection‐associated cancers in the year 2002. International journal of cancer, (2006); 118(12): 3030-3044.
- Kaae J, Hansen AV, Biggar RJ, Boyd HA, Moore PS, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. Journal of the National Cancer Institute, (2010); 102(11): 793-801.
- Lee S, Paulson KG, Murchison EP, Afanasiev OK, Alkan C, et al. Identification and validation of a novel mature microRNA encoded by the Merkel cell polyomavirus in human Merkel cell carcinomas. Journal of Clinical Virology, (2011); 52(3): 272-275.
- Bilal11 M, Ahmad A, Khan K, Ahmad N, Rehman AU, et al. Computational Prediction Of Micro-RNAs In Hepatitis B Virus Genome. J Appl Environ Biol Sci, (2014); 4(8S): 106-113.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0