Full Length Research Article
Antibacterial activity of leaf extracts of seven grape cultivars against six strains of bacteria
Naila Ali*, Humera Afrasiab, Sabahat Anwar
Adv. life sci., vol. 6, no. 4, pp. 159-164, August 2019
*- Corresponding Author: Naila Ali (Email: nailayawar23@gmail.com)
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Grape (Vitis spp.) is one of the most widely grown fruit crops in the world. It contains large amounts of phenolic compounds which have antimicrobial properties. Many efforts have been done to discover new antimicrobial compounds from various kinds of natural sources to benefit mankind. The current study is aimed to evaluate the antimicrobial properties of the methanolic extract of different cultivars of grapes against six bacterial strains.
Methods: In the present study, the antibacterial activity of different concentrations (25, 50 and 100mg/mL) of methanolic leaf extracts of seven cultivars of grape (Red Globe, Autumn Royal, Crimson, Thompson, Sundarkhani, Perlette and King’s Ruby) was tested against six strains of bacteria (Proteus sp., Streptococcus viridans, Escherichia coli, Pseudomonas sp., Staphylococcus aureus and Clostridium septicum) using agar well diffusion method.
Results: The methanolic leaf extract of all the cultivars showed significant antibacterial activity, suppressing the growth of all the six bacterial strains tested. The inhibition zones against the different bacteria ranged from 12.6 mm to 29.6 mm. The highest zones of inhibition were produced by Proteus sp. (29.6 mm), Pseudomonas sp. (27 mm), Staphylococcus aureus (27 mm), E. coli (26 mm), Streptococcus viridans (23.3 mm) and Clostridium septicum (21.0mm) at 100 mg/mL concentrations of different cultivars.
Conclusion: This study confirms that Vitis vinifera methanolic leaf extracts have inhibitory activity against all the tested microorganisms and is worthy of further investigation.
Keywords: Vitis vinefera; Antibacterial activity; Proteus sp.; Streptococcus viridans; E. coli; Pseudomonas sp.; MRSA; Clostridium septicum; Agar well diffusion
Grape is one of the most, economically important fruit crop and grown widely in the world [1, 2]. Hymas traced the usage of grapes to 7000BC and states that they were cultivated even afore the cereal [3]. De Candolle reported that the grapes were cultivated in Egypt around 4000BC [4].
Plants and their products are gaining interest for treating bacterial infections and also as food preservative and dietary supplements. Consumers are becoming more reluctant to use antibiotics and are interested in adopting plant derived medicines due to their lesser side effects. About 25-50% of current pharmaceutical drugs are derived from plants and plant products [5]. Several medicinal plants have been studied for their antimicrobial activities against different pathogenic bacterial species and many of them have also been found to be efficient against resistant microbial strains [6.7,8]. Plants are a rich source of secondary metabolites, like alkaloids, glycosides, terpenoids, saponins, steroids, flavonoids, tannins, quinones and coumarins and are a source of plant-derived antimicrobial substances [9]. These biomolecules produce antimicrobial activity through different mechanism. The saponins cause leakage of certain enzymes and proteins from the cell [10]. Terpenoids have the ability to dissolve cell wall of the microorganism by weakening the membranes [11]. Flavonoids are known to be synthesized in response to microbial infection by plants and have the ability to complex with bacterial cell walls [12]. Tannins form complexes with proline rich proteins and cause inhibition in the cell wall synthesis [13].
Although an average of 2-3 antibiotics derived from microorganisms are launched every year but their life span is very limited [14], and new alternative sources like plants must also be investigated. There are almost 390,000 species of plants on earth, and most remain to be assessed for their medicinal properties. In this work the antimicrobial activity of seven grape cultivars were screened against six bacterial strains.
Preparation of Plant leaves extract
Seven grape cultivars (Red Globe, Autumn Royal, Crimson, Thompson, Sundarkhani, Perlette and King’s Ruby) were grown in the Botanical Garden, University of the Punjab. Among them, Red Globe, Crimson and King’s Ruby were red grapes; Autumn Royal was purple while Thompson, Sundarkhani and Perlette were white table grape cultivars. Fully grown mature leaves from healthy one-year-old plants were taken during spring season for sample preparation. The extraction method was based on that described by Khamsah et al. [15]. From each cultivar, 100 gms of leaves were collected, thoroughly washed with dH2O, spread on paper sheets and allowed to dry for 3-4 days in shade. The dried samples were crushed into a fine powder and mixed into 100 mL of extracting solvent (methanol) and allowed to stand at room temperature
for 24 hours. Samples were then filtered using Whattman filter paper I. The Soxhlet method was performed for extraction [16]. The filtrate was then poured in a round bottom flask, placed on a rotary evaporator and left for 12 hours at 60ºC. The remaining methanol was removed by freeze drying and the samples were kept at 4ºC until use.
Cultures of bacterial strains
Six strains of bacteria, including three gram negative (Proteus sp., Escherichia coli, Pseudomonas sp.) and three gram positive (Streptococcus viridian, Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium septicum) were provided by the Department of Zoology, University of the Punjab, Lahore. These microorganisms were inoculated on nutrient agar (Merck) slants and preserved at 4°C for further use.
Inocula preparation
The inocula of bacterial strains were prepared following the growth method of Weigand et al., [17]. The test organisms were prepared by taking a loop full of culture and transferring them into nutrient agar medium to get a pure colony at 37 °C for 24 hours. Then 3-5 pure colonies were selected and transferred to 10mL nutrient broth and mixed well using a vortex mixer. The cultures were grown at 37°C for 8-12 hours until it reached a final concentration of 10 7- 10 8 CFU/mL.
Agar well dilution method
Each bacterial culture was taken and 0.5mL (1 to 2 x 104 CFU) of each was spread on nutrient agar plates. Wells of 6mm diameter were made by using sterile cork borer. Then 50 µl leaf extracts (25, 50 and 100 mg/mL) of each cultivar of grapes was poured into separate wells. These plates were then incubated at 37°C. After 4-5 days the diameter of inhibition zones was measured with a ruler and compared with the control well containing nutrient broth only.
Statistical Analysis
Each experiment was repeated three times. The results (zones of inhibition) were analyzed by analysis of variance (ANOVA) and the difference between means were scored using Duncan’s Multiple Range Test P ≤ 0.05 on the statistical package of SPSS (Version 12).
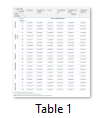
In the agar diffusion assay, the selected grape cultivars showed a broad-spectrum antibacterial activity against both gram positive and gram negative bacteria with zones of inhibition ranging from 12 mm to 29 mm. Among the three concentrations (25, 50 and 100 mg/mL) of the leaf extracts used, zone of inhibition shown by all the tested organisms increased with an increase in the concentration of leaf extracts and maximum inhibition was recorded at 100mg/L (Table 1). The methanol extract of Autumn Royal and Crimson cultivars showed the maximum zone of inhibition against Proteus sp. ranging from 21 mm to 29 mm followed by Staphylococcus aureus (MRSA) and Pseudomonas sp. while in others it ranged from 12 mm to 23 mm. E. coli was the most resistant strain of bacteria exhibiting 22 mm, 24.6 mm and 26 mm zone of inhibition followed by Pseudomonas sp. showing 19.2, 21.6 and 24mm inhibition zone at 25, 50 and 100 mg/mL concentrations of the leaf extract of Red Globe cultivar, respectively.
Antibacterial activity of the cultivar Thompson showed that Pseudomonas sp. was the most resistant strain at all the concentrations (25, 50 and 100 mg/mL) of the leaf extract exhibiting 21.3 mm, 24.3 mm and 26.0 mm zones of inhibition as compared to other bacterial strains tested. In Sundarkhani cultivar, maximum zone of inhibition ranging from 21 mm to 26.6 mm was recorded for Pseudomonas sp., Proteus sp. and E. Coli followed by other bacterial strains (Table 1).
Results of antibacterial activity suggested that E. coli was the most resistant strain to all the concentrations (25,50 and 100 mg/mL) of the leaf extract of Perlette cv. exhibiting 21.3, 22.3 and 25.6 mm zones of inhibition, respectively. Pseudomonas sp. and Proteus sp., showed zones of inhibition ranging from 19.6 to 23.3 mm in all the concentrations tried.
The most resistant bacterial strain to all the concentrations (25,50 and 100 mg/mL) of leaf extract of cultivar King’s Ruby was Staphylococcus aureus resulted in 22, 24.7 and 27 mm zones of inhibition, followed by E. coli, Proteus sp., Streptococcus viridans, Pseudomonas sp. and Clostridium septicum.
Among all the concentrations tried, maximum zone of inhibition of 29.6 mm at 100 mg/mL leaf extract of Autumn Royal was exhibited by Proteus sp. and least (15 mm) was showed by E. coli. Among others, minimum zone of inhibition (17 mm) was shown by Streptococcus viridins and Clostridium septicum from 100 mg/mL leaf extracts in Autumn Royal and in Crimson cultivar respectively (Table 1).
In the present work, the methanolic leaf extracts obtained from different cultivars of grapes showed strong activity against most of the tested bacterial strains. Among the different microorganisms tested, Proteus sp. proved to be the most resistant bacterial strain, followed E. coli, Staphylococcus aureus, and Pseudomonas sp., while Streptomyces viridans and Clostridium septicum showed smaller zones of inhibition as compared to others in all the cultivars. Similar work was reported by Ahmad et al. working on the antimicrobial activity of Vitis vinifera hot water leaf extract reported that the highest zone of inhibition was produced by S. aureus (30 mm), followed by E. faecalis (28.9 mm), E. coli (28 mm) and P. aeruginosa (23.7mm) [18]. Papadopoulou et al. demonstrated that S. aureus was most sensitive against antibacterial action of wine extracts [19]. While, Yadav et al. and Kabir et al. reported that S. typhimurium and E. coli were resistant against antimicrobial action of grape extracts [5, 20]. In general, the present study showed that gram negative bacteria exhibited maximum zone of inhibition as compared to gram positive bacteria except Staphylococcus aureus (MRSA) in all the concentrations of methanolic leaf extracts from all the cultivars tried. Nirmala and Narendhirakannan reported maximum inhibition in methanolic extracts of Muscat variety of grapes as compared to all others extracts (ethanolic, water and acetone) tried [21]. Similar results were also observed by other workers [22 - 24].The therapeutic value and bioactivity of medicinal plants depends upon the phytochemical constituents present in them. Grapes are among the fruits containing the highest content of phenolic substances, which vary from simple (monomers) to complex compounds (oligomers and polymers). Grape polyphenols contain from simple (monomers) to complex compounds (oligomers and polymers). Polyphenols are the most important phytochemicals in grape and are mostly negatively charged. The phenolic compounds include phenolic acids, flavonols, anthocyanins and stilbenes [25, 26, 27]. The pigment anthocyanins are the main polyphenolics in red grapes, while flavonols are mostly present in white varieties [28, 29, 30]. Flavonoids are widely distributed in grapes, especially in seeds and stems. As an antimicrobial agent, these polyphenols has the potential to penetrate the cell membrane and react with the cytoplasm or cellular proteins. Therefore, these highly negative charged antimicrobial polyphenolic compounds can be used for controlling the growth of pathogenic bacteria [31].
The antibacterial activity of the leaf extracts of grape cultivars as recorded in present study may therefore be attributed to the presence of flavonoids, tannins, phenolic compounds and other constituents present in the methanolic extract. Further studies are needed to isolate and characterize the bioactive principles for the development of new antibacterial drugs.
Acknowledgements
The authors are gratefully acknowledged to Higher Education Commission (HEC) Islamabad, for financial support and Department of Botany, University of the Punjab, Lahore for providing necessary laboratory facilities.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Mederos M. Culture medium requirements for micropropagation of Vitis vinifera L. cv. Listan Blanco. Acta Horticulturae, (2007); 754(1): 265-271.
- Kurmi US, Sharma DK, Tripathi MK, Tiwari R, Baghel BS, Tiwari S. Plant regeneration of Vitis vinifera (L) via direct and indirect organogenesis from cultured nodal segments. Journal of Agricultural Technology, (2011); 7(3): 721-737.
- Hymas E. Vineyards in England. 1954, (1954); Pp: 27-33. Feber and Feber Limited. London.
- De Candolle, A. Plants Cultivated for Their Seeds. In: Origin of Cultivated Plants, 2nd Edition. International Scientific Series. (1886), Vol. XLIX: 25-27, Kegan Paul Trench and Co-operation, London.
- Yadav D, Kumar A, Kumar P, Mishra D. Antimicrobial properties of black grape (Vitis vinifera L.) peel extracts against antibiotic-resistant pathogenic bacteria and toxin producing molds. Indian Journal of Pharmacology, (2015); 47(6): 663-667.
- Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethnopharmacology, (2001); 74(2):113–23.
- Aliyu A, Musa AM, Abdullah MS, Oyewale AO, Gwarzo US. Activity of plant extracts used in northern Nigerian traditional medicine against methicillin-resistant Staphylococcus Aureus (MRSA). Nigerian Journal of Pharmaceutical Sciences, (2008); 7(1):1–8.
- Suleman S, Alemu T. A survey on utilization of ethnomedicinal plants in Nekemte town, East Wellega (Oromia), Ethiopia. Journal of Herbs, Spices and Medicinal Plants, (2012); 18(1):34–57.
- Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agents: current methods and future trends. Journal of Medicinal Plants Research. (2010); 4:104–11.
- Zablotowicz RM, Hoagland RE, Wagner SC. Effect of saponins on the growth and activity of rhizosphere bacteria. Advances in Experimental Medicine and Biology, (1996); 405:83–95.
- Hemandez NE, Tereschuk ML, Abdala LR. Antimicrobial activity of flavonoids in medicinal plants from Tafí del Valle (Tucumán, Argentina) Journal of Ethnopharmacology, (2000); 73(1-2):317–322.
- Marjorie C. Plant products as antimicrobial agents. Clinical Microbiology Reviews, (1999); 12(4):564–582.
- Mamtha B, Kavitha K, Srinivasan KK, Shivananda PG. An in vitro study of the effect of Centella asiatica [Indian pennywort] on enteric pathogens. Indian Journal of Pharmacology, (2004); 36(1): 36–41.
- Cowan MM. Plant products as antimicrobial agents. Clinical Microbiological Reviews, (1999); 12(4): 564–582.
- Khamsah SM, Akowah G, Zhari I. Antioxidant activity and phenolic content of Orthosiphon stamineus benth from different geographical origin. Journal of Sustainable Science and Management, (2006); 1: 14-20.
- Siddhuraju P, Becker K. Antioxidant properties of various extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of Agricultural Food and Chemistry, (2003); 51: 2144-2155.
- Wiegand I, Hilpert K, Hancock RE. Agar and broth methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols, (2008), 3(2): 163-175.
- Ahmad W, Khan MI, Waqar M, Khan MA, , Khan A, Ramazan R, Wali S, Ahmad F, Khan N, Yousaf S, Zeb M, Khan A, Rahman M, Faisal S. In vitro antibacterial activity of Vitis vinifera leaf extracts against some pathogenic bacterial strains. Advances in Biological Research, (2014); 8(2): 62-67.
- Papadopoulou C, Soulti K, Roussis IG. Potential antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Biotechnology, (2005); 43:41-46.
- Kabir F, Sultana MS, Kurnianta H. Antimicrobial activities of grape (Vitis vinifera L.) pomace polyphenols as a source of naturally occurring bioactive components. African Journal of Biotechnology, (2015); 14: 2157-2161.
- Nirmala G, Narendhirakannan RT. In vitro antioxidant and antimicrobial activities of grapes (Vitis vinifera L) seed and skin extracts – Muscat variety. International Journal of Pharmaceutical Sciences, (2011); 3:242-249.
- Cheng VJ, Bekhit AE, McConnell M, Mros S, Zhao J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activity of extracts from wine residue from cool climate. Food Chemistry, (2012); 134: 474-482.
- Baydar NG, Ozkan G, Sagdic O. Total phenolic contents and antibacterial activities of grapes (Vitis vinifera L.) extracts. Food Control. (2004); 15: 335-339.
- Ozkan G, Sagdic O, Baydar NG, Kurumahmutoglu Z. Antibacterial activities and total phenolic contents of grape pomace extracts. Journal of Science, Food and Agriculture, (2004); 84:1807-1811.
- Dopico-Garcia MS, Fique A, Guerra L, Afonso JM, Pereira O, Valentao P, Andrade PB, Seabra RM. Principal components of phenolics to characterize red Vinho Verde grapes: anthocyanins or non-coloured compounds? Talanta, (2008); 75: 1190–1202.
- Novaka I, Janeiroa P, Serugab M, Oliveira-Brett AM. Ultrasound extracted flavonoids from four varieties of Portuguese red grape skins determined by reverse-phase high-performance liquid chromatography with electrochemical detection. Analytica Chimica Acta, (2008); 630: 107–115.
- Spacil Z, Novakova L, Solich P. Analysis of phenolic compounds by high performance liquid chromatography and ultra-performance liquid chromatography. Talanta, (2008); 76: 189–199.
- Chacona MR, Ceperuelo-Mallafrea V, Maymo-Masipa E, Mateo-Sanzb JM, Arolac L, Guitierreza C, Fernandez-Reald JM, Ardevolc A, Simona I, Vendrella J. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine, (2009); 47: 137–142.
- Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray CA, Kuszynski SS, Joshi HG. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology, (2000); 148: 187–197.
- Cantos E, Espin JC, Tomas-Barberan FA. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. Journal of Agricultural and Food Chemistry, (2002); 50: 5691–5696.
- Paulus W. Microbicides for the Protection of Materials – A Handbook. (1993). p.96. Chapman and Hall, London, UK.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0