Full Length Research Article
Amino acid consumption and secretion patterns of Staphylococcus aureus following growth in sub-optimal environmental conditions
Mousa M. Alreshidi
Adv. life sci., vol. 7, no. 2, pp. 98-105, February 2020
*- Corresponding Author: Mousa M. Alreshidi (Email: mousa.alreshidi@uon.edu.au )
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Staphylococcus aureus is highly associated with nosocomial infections due to its ability to adapt to wide range of environmental parameters. The aim of this study was to evaluate amino acids consumption and secretion by S. aureus at mid-exponential and stationary phases under growth in sub-optimal conditions, including changes in pH, temperature and osmolality.
Methods: The consumption and secretion of amino acids were determined by subtracting the original concentrations of the free amino acids in the media from those estimated at both mid-exponential and stationary phases of growth.
Results: The analysis revealed that the consumption and secretion profiles were substantially different between cells grown under optimal control conditions, when compared with those exposed to sub-optimal conditions. The analyses of the supernatants harvested at mid-exponential phase revealed that the total consumption of amino acids was increased by 1.2 and 1.7 times by cells grown at either pH 6 or 8 and 35°C with additional of 5 % NaCl, respectively. However, the final levels of amino acids consumed at stationary phase were significantly reduced in the cells grown in sub-optimal conditions compared with bacteria cells grown under optimal conditions.
Conclusion: It was evident that various environmental conditions led to differential profiles of amino acid consumption and secretion.
Keywords: Staphylococcus aureus; Amino acid metabolism; Stress responses
S. aureus is a vital bacterium skillful of producing an extensive variety of infections from minor skin to life-threatening sepsis and endocarditis [1]. S. aureus is capable to adjust their cytoplasmic composition following exposure to changes in the environmental factors such as variation in temperatures, pH and osmolality [2,3]. One of the main areas in microbiology is the investigation of bacterial acclimatization to changes in the environmental conditions, as the understanding of bacterial adaptation and survival mechanisms would pave ways to minimize infections [4,5]. Adaptation of bacteria depends on their capability to produce the optimum metabolic homeostasis vital for survival in response to undesirable environments [6-8]. Alterations to environmental influences can induce substantial adjustments in external morphology and lead to the development of biofilms [9-13].
Phenotypic shift such as small colony variants (SCVs) of S. aureus can be formed during the exposure to variations in temperatures, pH, salinity, and exposures to antibiotics, etc. [14-16]. SCVs are sub-population of bacteria that are categorized with reduced metabolism and enhanced resistance to antibiotics [16,17]. The growth of S. aureus in the presence of higher NaCl led to increased production of slime, associated with increase in the expression of biofilm genes such as the icaA gene [18]. Additional, biofilm can be developed following growth in other non-ideal growth conditions such as alkaline environments [19,20]. Both, SCV phenotypes and biofilm profound resistance and stable adhesion; which eventually assist bacteria to survive under alterations in the environments [21-24].
A prior study has revealed that specific amino acids were consumed by the biofilm cultures of S. aureus in comparison to planktonic cultures [25]. Hence, it was suggested that amino acid consumption is a vital for staphylococcal biofilm formation and adaptation to alterations in pH [12,26]. The absence of proline in the growth cultures reduced staphylococcal proliferation and survival [27]. similarly, combinations of subtle environmental conditions led to extensive alterations in amino acid uptake profiles [28]. Likewise, substantial alterations in cytoplasmic amino acid compositions of S. aureus in response to variation in temperature, pH and salinity were reported [3]. Different strains of S. aureus consumed different quantities of media metabolites [29] and S. aureus cultured with limited glucose also adjusted their consumptions of media metabolites including amino acids [30]. This was hypothesized as an acclimatization mechanism used by the bacteria to consume amino acids for energy and alternative resources to support growth based on nutrient accessibilities from the surrounding milieu [12,31]. To date, it has been established that the growth of S. aureus to subtle variations in environmental conditions including temperature, pH and NaCl led to substantial changes in metabolites, proteins and external morphological structures compared with those grown under ideal conditions [2,3,6,11,32]. It has also been shown that S. aureus taken up a significant amount of glycine when incubated in defined media supplemented with 5% NaCl compared to the cells grown in ideal conditions [2]. The current study investigated whether S. aureus could alter the amino acid consumption profiles from growth media in response to variations in environmental factors such as pH, temperature and NaCl when grown to mid-exponential and stationary phases. It was assumed that S. aureus would necessitate a prompt acclimatization to variations in the temperature, pH and osmolality that need simultaneous quick adjustments in the demand of biosynthesis of metabolites and proteins crucial for bacterial survival and evolution. Therefore, to do so, the bacterium would adjust its amino acid uptake profiles to achieve the ideal metabolism obligatory for acclimatization and survival following exposure to variations in the environmental parameters.
Bacterial strain and Growth conditions
The bacterial strain of S. aureus used in this study was isolated from a patient who had chronic muscle pain [33]. This strain used in the following studies to investigate metabolic and proteomic changes to environmental stresses [2,3,6]. The bacterium was maintained as culture stock on horse blood agar (HBA) and preserved appropriately on sterile glass beads at -80°C with a regular sub-culturing to maintain viability. The identity of the isolate was checked regularly using API™ Staph biochemistry and through PCR [34].
S. aureus was cultured in a sub-optimal of environmental conditions for analyses of amino acid consumption by S. aureus following growth in tryptic soy broth media (TSB) [3]. The reference control included cells grown under ideal conditions of pH7 at 37°C with no added NaCl in tryptic soy broth medium (TSB) and two sets of experimental conditions were applied with 1) pH6 at 35°C with 5% NaCl added in TSB; 2) 35°C and pH8 with 5% NaCl added in TSB.
An overnight starter culture (50 ml) of S. aureus was grown for 16h in Tryptic Soy Broth (TSB) at 37°C in an orbital shaking incubator) to be used as an inoculum for the growth experiments. Replicates of each condition containing 95 ml TSB culture media were inoculated with 5 ml of overnight culture in 500 ml conical flasks which were then grown until mid-exponential and stationary phases with constant agitation (120 rpm).
Amino acid consumption and secretion analysis
Five ml of tryptic soy broth media (TSB) prior and after the growth of S. aureus was collected at both mid-exponential and stationary phases. The collected supernatants were filtered through a Millex® membrane filter (0.22 mµ) and stored at -20 °C for further analysis. 100 µl of sterile TSB media and the filtered supernatants harvested at mid-exponential phase were diluted with 300 µl of sterile Milli-Q water, and the 100 µl harvested at stationary phase was diluted with 100 µl with Milli-Q water. The dilution was done to avoid overwhelming the analytical capacity of the gas chromatography column. An aliquot of 100 µl of diluted supernatants was then processed and analysed using a commercial analytical kit (Phenomenex® EZ: faast™). The technique was processed according to the manufacturer’s instructions. The derivatized amino acids were then analyzed using an Agilent gas chromatograph (Hewlett Packard HP 6890 series) coupled with flame ionization detector (GC-FID) which was calibrated to measure more than 40 amino acid metabolites as previously described [10]. The injection volume was 2 μl with spitless mode and flow rate of the carrier gas (Helium) was 0.5 ml/min. Nor-valine was used as an internal standard to calculate the concentrations of amino acids present in the sample as nmol/100 µl. The results were normalized by multiplied by dilution factors.
Statistical analyses
The consumption and secretion data produced by gas chromatography and flame ionization (GC‐FID) were assessed via ANOVA statistical analysis to detect the consumption and secretion of amino acids that were significantly altered after the exposure to variations in temperature, pH and NaCl (Statistica, TIBCO Software Inc. [2017], data analysis software system, version 13. http://statistica.io). Principal component analyses (PCA) were also conduct for the same data to further have an insight on the impact of alterations in the environmental conditions on amino acid consumption and secretion. The PCA model complexity and validity were evaluated by cross validation as applied in the software.
Amino acids consumption and secretion assessment at mid-exponential phase
Cultures of S. aureus were grown to mid-exponential phase of growth under various combinations of sub-optimal environmental parameters with temperature ranging from 35-37°C, pH 6-8 and higher NaCl (0-5%). The supernatants were harvested at mid-exponential phase to assess the consumption and secretion of amino acids. The amino acid consumption and secretion analyzed in each of the samples were reproducible within each experimental group with specific changes in the consumption and secretion of amino acids (Fig 1). The analysis of amino acid consumption in cells grown in ideal and non-ideal conditions revealed that leucine, lysine, Phenylalanine and serine were found to be the major amino acids consumed from the culture media. However, when bacteria were grown in altered environmental parameters, the consumption of leucine and lysine was greatly increased. Phenylalanine and serine did not change in treatment C, but significantly altered in experimental group B compared with controls. Various specific alterations in amino acid consumption and secretion associated with the different environmental conditions were apparent in Fig 1. When bacterial cells were exposed to altered environmental conditions, phenylalanine was the third most consumed amino acid after lysine and glutamic acid. Glutamine was significantly secreted in cells grown at lower temperature of 35 at pH8 with additional 5% NaCl. The total quantities of amino acids consumed was 1.2 and 1.7 times higher when bacterial cells were exposed to pH6 and 35 ◦C with additional of 5% NaCl in treatment B and pH 8 and 35 ◦C with 5% NaCl added in treatment C, respectively.
Principal component analysis (PCA) indicated that major differences between the amino acid compositions of the culture supernatants from bacterial cells grown under normal conditions (A) and those grown at different treatment regimens (B and C) (Fig 2-A). The analysis of the PCA revealed that the cells exposed to pH 6 and 35 °C with the additional of 5%NaCl (B) were the most separate treatment from the control (A) and other treatment group (C). It appeared that bacteria cells grown at lower pH (6) and 35◦C with additional of 5% NaCl (B) had more separable amino acid consumption profiles compared with equivalent cells grown with more alkaline conditions (C).The loading scatterplot showed the consequence of the variables (amino acids) on PCA graph separation and clustering of the treatments (Fig 2-B).
Amino acids consumption and secretion assessment at stationary phase of growth
Cultures of S. aureus exposed to the same environmental conditions A-C, were harvested at the stationary phase of growth to evaluate the impact on the total consumption and secretion of amino acids during culture growth. The total quantities of amino acids consumed was 2.6 and 3.1 times lower when bacterial cells were grown under more acidic (B) or alkali (C) conditions and lower temperature of 35C with 5% NaCl added, respectively. However, the total amounts of amino acids secreted was 8.1 and 39.3 times higher when S. aureus was grown in pH 6 or 8 at 35 ◦C with 5% NaCl added, respectively. Lysine, leucine, and serine continued to be the most consumed amino acids from the growth media when S. aureus grown to stationary phase in normal conditions (Fig 3). However, alanine, serine and glutamic acid became the major amino acids consumed in treatment B and C. The profile of amino acid consumption and secretion was differential between the experimental regimes with numerous substantial differences observed between utilization rates of the amino acids.
Amino acids consumption and secretion data were applied to multivariate analysis using PCA. PCA plot showed significant differences between the amino acid compositions of the culture supernatants from the different experimental groups (Fig 4-A). The analysis revealed an obvious separation of reference control and treatment samples due to their amino acid consumption and secretion profiles analyzed at stationary phase of growth. It was also evident from PCA plot that cells gown with additional of 5% NaCl at 35 °C and pH 6 or 8 (B and C) had very different amino acid consumption and secretion profiles compared to equivalent cells grown at ideal conditions (A). The loading scatterplot resultant from PCA scores indicated the amino acid consumption and secretion that contributed to the importance of the clustering/separation observed on the PCA plot (Fig 4-B).
Comparison of mid-exponential vs. stationary phase amino acids consumption and secretion profiles
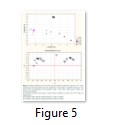
The amino acid compositions of S. aureus culture supernatants from the cells grown under normal conditions (A) and those exposed to altered environmental conditions (B and C) were compared between the mid-exponential and stationary phases of growth using PCA. The PCA analysis rendered a two-component model as validated by cross-validation (CV). The PCA scores for mid-exponential and stationary phases exhibited two obvious clusters significantly separated by component 1 scores where the amino acid consumption and secretion data harvested at the mid-exponential phase had a negative component 1 score and those collected at the stationary phase had positive component 1 scores (Fig 5-A). Explanation of 90% of the data was accomplished (i.e., R2 = 0.50 and Q2 = 0.28) and the eigenvalue for component 1 was 10.5 as compared to 5.3 for component 2. The loading scatterplot indicated the significance of key variables on the clustering and separation. The stationary phase was described via a greater consumption of amino acids from the medium by cells grown at normal conditions, whereas the mid-exponential phase control cells were characterized by consumed valine and tyrosine in high levels before secreting these amino acids back into the medium by the time of analysis at stationary phase.
Figures & Tables
The outcomes from the present investigation revealed that the profiles of amino acid consumption and secretion activities noted in S. aureus at the mid-exponential and stationary phases of growth were different from cells grown at ideal conditions and those grown under altered environmental conditions. It was obvious that the demands for amino acids adjusted following exposure to variations in the environmental factors involving pH, temperature and osmolality. Amino acids are important nutrients for cell structure and metabolic regulation as in addition to their use in protein synthesis [35-37]. The availability of free amino acids in the growth medium is important for optimal growth capacity under ideal and non-ideal environmental conditions [38].
The growth of S. aureus in a combination of environmental conditions including variation in pH, temperature and NaCl caused substantial differences in the consumption of amino acids by bacterial cells harvested at mid-exponential phase. The consumption of amino acids at different rates possibly reflected difference metabolic demands. The exposure of S. aureus to the same environmental conditions led to different cytoplasmic amino acid compositions compared with reference controls [3]. This increase in the consumption of amino acids is concomitant with the increase of the cytoplasmic amino acids, indicating that the bacterium had to consume higher levels of amino acids to adapt to the changes in temperature, pH and osmolality [39-41].
The analysis of consumption and secretion patterns of amino acids by S. aureus at the stationary phase following exposure to alterations in environmental conditions revealed extraordinary amino acid consumption and secretion profiles, suggesting that the bacterium adjusted its amino acid consumption to obtain optimal metabolism associated with the external environmental factors. The total amount of amino acids taken up at the stationary phase measurements were significantly reduced in treatment regimens compared with reference controls. Stationary phase is a complex environment including reduced amounts of nutrients, buildup of metabolic waste products and alterations in pH values [42,43]. The variation in the consumption of amino acids could suggest that alternative mechanisms were required by bacterial cells under each treatment regimens to obtain the optimum metabolic homeostasis essential for survival. Reduced amino acids consumption at stationary by bacterial cells exposed to sub-optimal environmental conditions may represent a reduced metabolic rates in the cytoplasm. This would represent an important way in adapting the cellular homeostasis under alterations in the environmental conditions [44,45].
The significant secretion of amino acids by cells grown in sub-optimal conditions in current study may be associated with peptidoglycan synthesis and other components in the cell wall [46,47]. The secretion of amino acids into the external media could be a mechanism to facilitate the adaption processes to encounter the osmotic pressure implemented by the additional of 5% NaCl to the growth media. Cultures of stationary phase secreted high levels of many amino acids including valine, lysine, ornithine, tyrosine and tryptophan. It has been reported that ornithine was significantly released by staphylococcal biofilm cultures into the external media [12]. In the same manner valine was released by S. aureus COL following incubation in eukaryotic cell media [29]. Many bacteria release D-amino acids at a stationary phase into external media, which have a great impact on the cell wall structure [47]. Therefore, the secretion of these amino acids might be adaptive machinery for bacteria to the changing in environmental conditions [48].It has been shown that bacteria secreted small molecules in the stationary phase of growth [49,50]. These molecules have very important roles as signaling molecules for cell-cell communications or preventing the growth of other microorganisms sharing the same locations [51]. Therefore, the secretion of specific amino acids could be an indication of the initiation of biofilm formation at stationary phase [26,52]. In the stationary phase, remarkable changes occur involving structural, physiological alteration and DNA/protein ratio was reported to be greater [19,42].
A significant synthesis of polysaccharide intercellular adhesin in biofilm cultures has been related with repressed tricarboxylic acid activity [53]. Hence, this may illustrates the substantial decrease in amino acid consumption in the stationary phase, as it possibly links with alterations in morphological structures such as cell size, phenotypic shift and biofilm development [9,10]. It has been shown that SVCs have a reduced metabolic activity with a decrease of toxins productions. The activity of tricarboxylic acid cycle (TCA) was highly associated with polysaccharide intercellular adhesin (PIA) production biofilm [35,53-55], it showed that active TCA led to repression of PIA production and hence reduced the synthesis of virulence factors [56]. Therefore, it has been suggested that the uptake of amino acids would be critical for TCA activation and therefore, pathogenicity. Metabolomic studies have demonstrated that amino acid catabolism is important for the synthesis of intermediates oxaloacetate, oxoglutarate, phosphenpyuvate and pyruvate for TCA and gluconeogenesis [53,57,58] . On the basis of these results, it has been suggested that the bacterium regularly detecting and adapting to the changes in the external environmental conditions by inducing the best operative and competent phenotypes for survival in stressed environments [9,10,59,60]. It is still remain unclear why some amino acids would be initially consumed during growth to the mid-exponential phase and then secreted at high amounts by stationary phase. The concentrations of these amino acids in the external growth media at stationary phase indicated that significant amounts of these amino acids were produced de novo.
The study of amino acid consumption and secretion by S. aureus under sub-optimal conditions would be very essential to control bacterial adaptation and survival in response to subtle alterations in the environmental parameters. The data of this investigation revealed that S. aureus induced a remarkable modification in amino acid consumption and secretion patterns following the exposure to changes in the environmental conditions. In addition, specific alterations in amino acid consumption and secretion would indeed form the basis for acclimation that could induce evolutionary survival and optimal adaption of this bacterium. It is clear that certain strategies were initiated to adapt during the growth in non-ideal environmental condition to acquire optimal metabolism status by extracting specific quantities of amino acids from growth media. It was thus concluded that these alterations in the amino acid consumption and secretion patterns are vital for the adaptation processes following exposure to undesirable conditions.
Authors' Contribution
This is a single author study. MMA conceptualized, designed, experimented, acquired data, analysed & interpreted data and drafed the manuscript.
Funding
This work was supported by the University of Ha’il (grant No. 160967).
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Alreshidi MM, Dunstan RH, Onyango LA, Roberts TK (2013) Staphylococcal phenomics: metabolomic and proteomic responses to environmental stessors. In: Mendez-Vilas A, editor. Microbial pathogens and strategies for combating them: science, technology and education: Spain Formatex Research Center. pp. 690-701.
- Alreshidi MM, Dunstan RH, Macdonald MM, Smith ND, Gottfries J, et al. Amino acids and proteomic acclimation of Staphylococcus aureus when incubated in a defined minimal medium supplemented with 5% sodium chloride. Microbiologyopen, (2019); 8(6): e00772.
- Alreshidi MM, Dunstan RH, Gottfries J, Macdonald MM, Crompton MJ, et al. Changes in the Cytoplasmic Composition of Amino Acids and Proteins Observed in Staphylococcus aureus during Growth under Variable Growth Conditions Representative of the Human Wound Site. PLoS One, (2016); 11(7): e0159662.
- Onyango LA, Alreshidi MM. Adaptive Metabolism in Staphylococci: Survival and Persistence in Environmental and Clinical Settings. Journal of Pathogens, (2018); 1092632.
- Thompson KM, Jefferson KK (2009) Adaption ot Stress: Biofilms and Small-Colony Variants. In: 2, editor. Staphylococci in Human Diseases. pp. 109-110.
- Alreshidi MM, Dunstan RH, Macdonald MM, Smith ND, Gottfries J, et al. Metabolomic and proteomic responses of Staphylococcus aureus to prolonged cold stress. Journal of Proteomics, (2015); 12: 144-55.
- Tsai M, Ohniwa RL, Kato Y, Takeshita SL, Ohta T, et al. Staphylococcus aureus requires cardiolipin for survival under conditions of high salinity. BMC Microbiology, (2011); 11: 13.
- Rode TM, Moretro T, Langsrud S, Langsrud O, Vogt G, et al. Responses of Staphylococcus aureus exposed to HCl and organic acid stress. Canadian journal of microbiology, (2010); 56(9): 777-792.
- Onyango LA, Hugh Dunstan R, Roberts TK, Macdonald MM, Gottfries J. Phenotypic variants of staphylococci and their underlying population distributions following exposure to stress. PLoS One, (2013); 8(10): e77614.
- Onyango LA, Dunstan RH, Gottfries J, von Eiff C, Roberts TK. Effect of Low Temperature on Growth and Ultra-Structure of Staphylococcus spp. PloS One, (2012); 7(1): e29031.
- Crompton MJ, Dunstan RH, Macdonald MM, Gottfries J, von Eiff C, et al. Small changes in environmental parameters lead to alterations in antibiotic resistance, cell morphology and membrane fatty acid composition in Staphylococcus lugdunensis. PLoS One, (2014); 9(4): e92296.
- Zhu Y, Weiss EC, Otto M, Fey PD, Smeltzer MS, et al. Staphylococcus aureus biofilm metabolism and the influence of arginine on polysaccharide intercellular adhesin synthesis, biofilm formation, and pathogenesis. Infection and Immunity, (2007); 75(9): 4219-4226.
- Valle J, Da Re S, Schmid S, Skurnik D, D'Ari R, et al. The amino acid valine is secreted in continuous-flow bacterial biofilms. Journal of Bacteriology, (2008); 190(1): 264-274.
- Sendi P, Proctor RA. Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends in Microbiology, (2009); 17(2): 54-58.
- Schmitz FJ, von Eiff C, Gondolf M, Fluit AC, Verhoef J, et al. Staphylococcus aureus small colony variants: rate of selection and MIC values compared to wild-type strains, using ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin. Clinical Microbiology and Infection, (1999); 5(6): 376-378.
- Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nature Reviews Microbiology, (2006); 4(4): 295-305.
- von Eiff C, Proctor RA, Peters G. Small colony variants of Staphylococci: a link to persistent infections. Berliner und Münchener tierärztliche Wochenschrift, (2000); 113(9): 321-325.
- Lee S, Choi KH, Yoon Y. Effect of NaCl on Biofilm Formation of the Isolate from Staphylococcus aureus Outbreak Linked to Ham. Korean Journal for Food Science of Animal Resources, (2014); 34(2): 257-261.
- Jaishankar J, Srivastava P. Molecular Basis of Stationary Phase Survival and Applications. Frontiers in Microbiology, (2017); 82000.
- Jones EM, Cochrane CA, Percival SL. The Effect of pH on the Extracellular Matrix and Biofilms. Advances in Wound Care (New Rochelle), (2015); 4(7): 431-439.
- Li W, Li Y, Wu Y, Cui Y, Liu Y, et al. Phenotypic and genetic changes in the life cycle of small colony variants of Salmonella enterica serotype Typhimurium induced by streptomycin. Annals of Clinical Microbiology and Antimicrobials, (2016); 15(1): 37.
- Yao YF, Sturdevant DE, Otto M. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: Insights into the pathophysiology of S-epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. Journal of Infectious Diseases, (2005); 191(2): 289-298.
- Noumi E, Merghni A, M MA, Haddad O, Akmadar G, et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules, (2018); 23(10).
- Noumi E, Snoussi M, Alreshidi MM, Rekha PD, Saptami K, et al. Chemical and Biological Evaluation of Essential Oils from Cardamom Species. Molecules, (2018); 23(11).
- Ammons MC, Tripet BP, Carlson RP, Kirker KR, Gross MA, et al. Quantitative NMR metabolite profiling of methicillin-resistant and methicillin-susceptible Staphylococcus aureus discriminates between biofilm and planktonic phenotypes. Journal of Proteome Research, (2014); 13(6): 2973-2985.
- Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, et al. Global gene expression in Staphylococcus aureus biofilms. Journal of Bacteriology, (2004); 186(14): 4665-4684.
- Schwan WR, Wetzel KJ, Gomez TS, Stiles MA, Beitlich BD, et al. Low-proline environments impair growth, proline transport and in vivo survival of Staphylococcus aureus strain-specific putP mutants. Microbiology, (2004); 150(Pt 4): 1055-1061.
- Murphy GR, Dunstan RH, Macdonald MM, Gottfries J, Roberts TK. Alterations in amino acid metabolism during growth by Staphylococcus aureus following exposure to H2O2 – A multifactorial approach. Heliyon, (2018); 4(5): e00620.
- Dorries K, Lalk M. Metabolic footprint analysis uncovers strain specific overflow metabolism and D-isoleucine production of Staphylococcus aureus COL and HG001. PLoS One, (2013); 8(12): e81500.
- Liebeke M, Dorries K, Zuhlke D, Bernhardt J, Fuchs S, et al. A metabolomics and proteomics study of the adaptation of Staphylococcus aureus to glucose starvation. Molecular Biosystems, (2011); 7(4): 1241-1253.
- Stipetic LH, Dalby MJ, Davies RL, Morton FR, Ramage G, et al. A novel metabolomic approach used for the comparison of Staphylococcus aureus planktonic cells and biofilm samples. Metabolomics, (2016); 1275.
- Wehrli PM, Lindberg E, Svensson O, Sparén A, Josefson M, et al. Exploring bacterial phenotypic diversity using factorial design and FTIR multivariate fingerprinting. Chemometrics, (2014); 30283-289.
- Butt HL, Dunstan RH, McGregor NR, Roberts TK, Zerbes M, et al. An association of membrane-damaging toxins from coagulase-negative staphylococci and chronic orofacial muscle pain. Journal of Medical Microbiology, (1998); 47(7): 577-584.
- Brown GK, Martin AR, Roberts TK, Aitken RJ. Detection of Ehrlichia platys in dogs in Australia. Australian Veterinary Journal, (2001); 79(8): 554-558.
- Zhu Y, Xiong YQ, Sadykov MR, Fey PD, Lei MG, et al. Tricarboxylic acid cycle-dependent attenuation of Staphylococcus aureus in vivo virulence by selective inhibition of amino acid transport. Infection and Immunity, (2009); 77(10): 4256-4264.
- Turner GW, Cuthbertson DJ, Voo SS, Settles ML, Grimes HD, et al. Experimental sink removal induces stress responses, including shifts in amino acid and phenylpropanoid metabolism, in soybean leaves. Planta, (2012); 235(5): 939-954.
- Tremaroli V, Workentine ML, Weljie AM, Vogel HJ, Ceri H, et al. Metabolomic investigation of the bacterial response to a metal challenge. Applied and environmental microbiology, (2009); 75(3): 719-728.
- Dickgiesser N, Eppli P. Amino acid requirements of Staphylococcus aureus strains from Germany and Austria that produce toxic shock syndrome toxin-1 (TSST-1). Zentralbl Bakteriol Mikrobiol Hyg A, (1988); 268(1): 1-7.
- Liebeke M, Lalk M. Staphylococcus aureus metabolic response to changing environmental conditions – a metabolomics perspective. International Journal of Medical Microbiology, (2014); 304(3-4): 222-229.
- Richardson AR, Dunman PM, Fang FC. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Molecular microbiology, (2006); 61(4): 927-939.
- Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiological reviews, (1985); 49(4): 359-378.
- Nystrom T. Stationary-phase physiology. Annual Review of Microbiology, (2004); 58161-181.
- Aertsen A, Michiels CW. Stress and how bacteria cope with death and survival. Critical Reviews in Microbiology, (2004); 30(4): 263-273.
- Kussell E, Kishony R, Balaban NQ, Leibler S. Bacterial persistence: a model of survival in changing environments. Genetics, (2005); 169(4): 1807-1814.
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science, (2004); 305(5690): 1622-1625.
- De Kievit TR, Gillis R, Marx S, Brown C, Iglewski BH. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Applied and Environmental Microbiology, (2001); 67(4): 1865-1873.
- Aliashkevich A, Alvarez L, Cava F. New Insights Into the Mechanisms and Biological Roles of D-Amino Acids in Complex Eco-Systems. Frontiers in Microbiology, (2018); 9683.
- Lam H, Oh DC, Cava F, Takacs CN, Clardy J, et al. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science, (2009); 325(5947): 1552-1555.
- Martin MF, Liras P. Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annual Review of Microbiology, (1989); 43173-206.
- Lopez D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harbor Perspectives in Biology, (2010); 2(7): a000398.
- Lopez D, Kolter R. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiology Reviews, (2010); 34(2): 134-149.
- Resch A, Leicht S, Saric M, Pasztor L, Jakob A, et al. Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics, (2006); 6(6): 1867-1877.
- Vuong C, Kidder JB, Jacobson ER, Otto M, Proctor RA, et al. Staphylococcus epidermidis polysaccharide intercellular adhesin production significantly increases during tricarboxylic acid cycle stress. Journal of Bacteriology, (2005); 187(9): 2967-2973.
- Sadykov MR, Zhang B, Halouska S, Nelson JL, Kreimer LW, et al. Using NMR metabolomics to investigate tricarboxylic acid cycle-dependent signal transduction in Staphylococcus epidermidis. The Journal of Biological Chemistry, (2010); 285(47): 36616-36624.
- Sadykov MR, Olson ME, Halouska S, Zhu Y, Fey PD, et al. Tricarboxylic acid cycle-dependent regulation of Staphylococcus epidermidis polysaccharide intercellular adhesin synthesis. Journal of Bacteriology, (2008); 190(23): 7621-7632.
- Somerville GA, Proctor RA. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiology and Molecular Biology Reviews, (2009); 73(2): 233-248.
- Fleury B, Kelley WL, Lew D, Gotz F, Proctor RA, et al. Transcriptomic and metabolic responses of Staphylococcus aureus exposed to supra-physiological temperatures. BMC Microbiology, (2009); 976.
- Chatterjee I, Somerville GA, Heilmann C, Sahl HG, Maurer HH, et al. Very low ethanol concentrations affect the viability and growth recovery in post-stationary-phase Staphylococcus aureus populations. Appl Environ Microbiol, (2006); 72(4): 2627-2636.
- Casadevall A. Evolution of intracellular pathogens. Annual review of microbiology, (2008); 6219-33.
- de Jonge BL, Chang YS, Gage D, Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. The Journal of biological chemistry, (1992); 267(16): 11248-11254.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0