![]()
Yield potential study of Capsicum annuum L. under the application of PGPR
Muhammad Tariq1, Qurban Ali1, Anwar Khan1, Ghazanfar Ali Khan3, Bushra Rashid1, Muhammad Sarwar Rahi2, Arfan Ali1, Idrees Ahmad Nasir1, Tayyab Husnain1
Adv. life sci., vol. 1, no. 4, pp. 202-207, August 2014
*Corresponding Author: Anwar Khan (Email: anwarleo2003@gmail.com)
Author Affiliations
2- FMC United Pvt Limited, Lahore – Pakistan
3- Development and Planning Wing, Department of Agriculture Punjab, Lahore – Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Plant growth promoting bacteria (PGPR) play an important role in the healthy growth and yield improvement of different crops. The use of PGPR includes various groups of bacteria that live freely in the soil and has ability to enhance the growth of various crops through diverse mechanisms. This study was conducted to evaluate the yield enhancing effect of PGPR on Bell Pepper (Capsicum annuum).
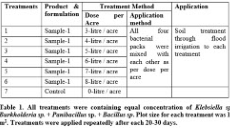
Methods: This study was conducted in the experimental fields of FMC United (Pvt) Limited at Sahiwal, Pakistan during the winter under controlled tunnel in the cropping season 2013-14. The various formulations of PGPR (Klebsiella sp. + Burkholderia sp. + Panibacillus sp. + Bacillus sp.) was applied after every 20-30days interval in the field of Bell Pepper to study their effects on per acre yield. Data was recorded and statistically analyzed to evaluate effects of PGPR on bell pepper yield.
Results: Results showed consistent per acre yield increase with the increase of PGPR formulations. Significant genotypic and phenotypic correlations were also found between yield per treatment and yield per acre. Higher yield per treatment and yield per acre was recorded at 6-litre/acre application of PGPR formulation.
Conclusion: It was concluded that use of PGPR could be helpful to improve the health of crop with increased yield of this important vegetable. It is proposed that further evaluation at multiple locations and compositions will help to chalk out a comprehensive application protocol of PGPR bacteria on vegetable as well as field crops.
Introduction
Bell pepper (Var. Grossum 2n=24) also known as "Sagia Mirch", Sweet pepper, Bullnose pepper, "Shimla Mirch" and Bell pepper is one of the popular vegetables in South Asia. Bell pepper contains large amount of vitamin A, C, pungency, colour and is also famous for its pleasant flavour and delicate taste [1, 2]. Bell pepper pungent is used in the formation of balms and carotenoids pigment (colour extract) is used as colour additives in poultry, food industry and prawn feed industry [3]. There are various ways to improve yield of crop plants. The use of plant growth promoting bacteria (PGPR) including various groups of soil based bacteria that live freely and have ability to promote the growth of crop plants through facilitating the resource acquisition and inhibiting the plant pathogen e.g. fungi [4,5]. The PGPR bacteria played an important role in the establishment of micro-biological niches in plant based systems in the rhizosphere portion of plant in contact with plant soil and roots. The plant roots exude a large number of organic nutrients like organic acids, sugars, vitamins, phytosiderophores, amino acids, nucleosides, mucilage and plant metabolizes that attract the micro-organisms through signals of their release in the soil and microbial habitat [5,6]. PGPR bacteria can increase the plant nutrition availability through the association of plant and PGPR like phosphate solubilization, nitrogen fixation and phytosiderophores [7]. The root activity of crop plant may be enhanced through the process of phytosiderophore, enzymatic activities, and rhizobial or mycorrhizal symbioses [8, 9]. The present study was conducted to evaluate the effect of PGPR on yield of bell pepper.
Methods
Sampling and isolation of PGPR
The rhizosphere soil samples were collected from sugarcane (Saccharum officinarum. L) plants of different farmer fields at Lahore, Pakistan during March, 2014. Healthy plants from the sugarcane field were selected and dug out with roots having soil. Roots and adhering soil samples were collected in sterile polythene bags, stored in ice container and transferred to laboratory for bacterial isolation, characterization and preservation. From each sample, ten grams of soil along with roots was crushed in sterile mortar and pestle in 10 ml autoclaved saline solution (0.85% NaCl). Serial dilutions (10−1 to 10−6) were prepared in autoclaved saline. To check the P solubilize characteristics of the PGPR, 100 µl was taken from dilution and streaked upon National Botanical Research Institute’s phosphate (NBRIP) media agar plates [10]. Plates were incubated at 28°C for 48 to 72 hours. Incubated plates were observed after 24 to 72 hours continuously, halo zones were observed in few colonies which could be the sign that phosphorous solubilizing bacteria (PSB) are present in the colonies. Those halo zone forming colonies were purified on NBRIP media. Different strains of PSBs found for whom glycerol stocks were prepared. Presumptive PSB were tested on plant in field to confirm and validate their phosphate solubilization activity.
Field Trial/Treatments
Field trial was conducted at experimental field of the M/s FMC United Pvt Limited at Sahiwal, Pakistan under controlled tunnel on Bell pepper during November 2013- February 2014. The experiment was designed according to the randomized complete block design that consists of seven treatments with three replications.
Statistical analysis
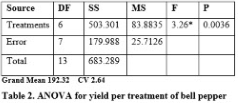
Data was recorded for yield per treatment and yield per acre. Analysis of variance [11] for phenotypic (rp) and genotypic (rg) correlation coefficient was calculated from that data as outlined by Kwon and Torrie [12]. Standard error of genotypic correlation coefficients (SE of rg) were calculated according to Reeve [13].
Results
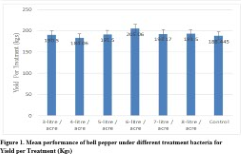
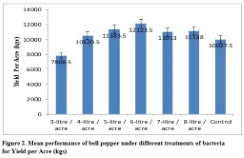
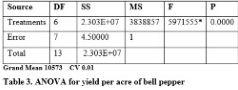
Significant differences were found for yield per treatment and yield per acre (Table 2 & 3). Increased yield per treatment (205.06 kg) and yield per acre (12124 kg) was recorded by applying 6-litres/acre when compared with yield per treatment (184.06 kg) under application of 4-litres/acre and yield per acre (7808.5 kg) with 3-litres/acre supplementation (Figure 1 & 2). The high heritability and genetic advance was found for yield per acre when compared with moderate heritability and genetic advance for yield per treatment (Table 3). Significant genotypic and phenotypic correlation between yield per treatment and yield per acre was established (Table 4). Bi-plot analysis revealed that the use of PGPR at 6-litre/acre was highly effective to improve yield per treatment and yield per acre while application of 3-litre/acre provided minimum yield per treatment and yield per acre. The other treatments provided average yield per treatment and yield per acre.
Tables & Figures
Discussion
It is suggested that the application of PGPR bacteria up to 6-litres/acre could enhance the yield of bell pepper per treatment and per acre. Analysis of higher genetic advance helped in better selection of synthetic variety for higher fruit yield while higher heritability suggested that hybrid development might be processed to improve yield per plant. Significant genotypic correlation indicated that the selection for higher yielding per acre may be attained on the basis of yield per treatment [13-15]. The use of PGPR bacteria to improve yield of bell pepper may also be helpful to improve yield in other crop plants. The significant phenotypic correlation suggested that the effect of PGPR bacteria on bell pepper was found to be significant role in improvement of pepper yield [8,9,16]. It was suggested that by using PGPR bacteria the overall performance of crop plants may be enhanced. It was also suggested fro study that there is need to use PGPR for evaluation in other crops like maize, wheat, rice, barley etc.
References
- Kaur C, Kapoor HC. Anti‐oxidant activity and total phenolic content of some Asian vegetables. International Journal of Food Science & Technology, (2002); 37(2): 153-161.
- Nadeem M, Anjum FM, Khan MR, Saeed M, Riaz A. Antioxidant Potential of Bell Pepper (Capsicum annum L.) a Review. Pakistan Journal of Food Science, (2011); 21(1-4): 45-51.
- Holguin G, Glick B. Transformation of Azospirillum brasilense Cd with an ACC deaminase gene from Enterobacter cloacae UW4 fused to the Tet r gene promoter improves its fitness and plant growth promoting ability. Microbial ecology, (2003); 46(1): 122-133.
- Hontzeas N, Saleh SS, Glick BR. Changes in gene expression in canola roots induced by ACC-deaminase-containing plant-growth-promoting bacteria. Molecular plant-microbe interactions, (2004); 17(8): 865-871.
- Drogue B, Combes-Meynet E, Moënne-Loccoz Y, Wisniewski-Dyé F, Prigent-Combaret C. Control of the cooperation between plant growth-promoting rhizobacteria and crops by rhizosphere signals. Molecular Microbial Ecology of the Rhizosphere, Two Volume Set, (2013); 281.
- Orhan E, Esitken A, Ercisli S, Turan M, Sahin F. Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Scientia Horticulturae, (2006); 111(1): 38-43.
- Badri DV, Weir TL, Van Der Lelie D, Vivanco JM. Rhizosphere chemical dialogues: plant–microbe interactions. Current opinion in biotechnology, (2009); 20(6): 642-650.
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annual review of microbiology, (2009); 63541-556.
- Couillerot O, Prigent‐Combaret C, Caballero‐Mellado J, Moënne‐Loccoz Y. Pseudomonas fluorescens and closely‐related fluorescent pseudomonads as biocontrol agents of soil‐borne phytopathogens. Letters in applied microbiology, (2009); 48(5): 505-512.
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS microbiology Letters, (1999); 170(1): 265-270.
- Kwon S, Torrie J. Heritability and interrelationship among traits of two soybean populations. Crop Sci, (1964); 4(2): 196-198.
- Reeve E. The variance of the genetic correlation coefficient. Biometrics, (1955); 11(3): 357-374.
- Ali Q, Ahsan M, Tahir MHN, Elahi M, Farooq J, et al. Genetic Variability for Grain Yield and Quality Traits in Chickpea.
- Ali Q, Elahi M, Ahsan M, Tahir MHN, Basra SMA. Genetic evaluation of maize (Zea mays L.) genotypes at seedling stage under moisture stress. International Journal for Agro Veterinary and Medical Sciences, (2011); 5(2): 184-193.
- Waseem M, Ali Q, Ali A, Samiullah TR, Ahmad S, et al. Genetic analysis for various traits of Cicer arietinum under different spacing. Life Sci J, (2014); 11(12s): 14-21.
- Ali Q, Ali A, Waseem M, Muzaffar A, Ahmad S, et al. Correlation analysis for morpho-physiological traits of maize (Zea mays L.). Life Science Journal, (2014); 11(12s).