![]()
Isolation, identification and in-vitro antibiotic sensitivity pattern of citrus canker causing organism Xanthomonas axonopodis
Mohammed Amirul Islam1, Reaz Mohammad Mazumdar2*, Saiful Islam2, Md. Jahangir Alam1, Samsed Ahmed Urmee1
Adv. life sci., vol. 1, no. 4, pp. 215-222, August 2014
*- Corresponding Author: Reaz Mohammad Mazumdar (Email: reazbio@gmail.com)
Author Affiliations
2- BCSIR Laboratories Chittagong, Bangladesh Council of Scientific and Industrial Research, Chittagong 4202, Bangladesh
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Xanthomonas axonopodis or X. axonopodis is the devastating causal organism of citrus canker, widely spread bacterial disease of plants from both epidemiological and economic points of view. Furthermore, the situation is worsening by the advent of increased antibiotic resistance among this bacteria. The major interests of this study were isolation, identification and in vitro antibiotic sensitivity pattern of the causal organism. Besides, herbal sensitivity of those organisms was also tested.
Methods: In this study, 9 isolates of the organism were identified based on morphological, cultural and biochemical characteristics. All the isolates were tested for antibiotic sensitivity against 5 commonly used antibiotics namely, cefotaxime, bacitracin, chloramphenicol, streptomycin and gentamycin.
Results: X. axonopodis was found 100% resistant to cefotaxime and 77.77% to bacitracin. Chloramphenicol was found most effective as all the isolates were sensitive to it. The herbal sensitivity of X. axonopodis was tested with the plant extract of Allium cepa, Allium sativum, Litchi chinensis, Vitis amurensis and Syzygium cumini. Among the plant extracts, the pathogens were found most sensitive to Allium sativum and Syzygium cumini and resistant to V. amurensis.
Conclusion: The study showed herbal treatment can be implicated for the disease citrus canker caused by antibiotic resistant X. axonopodis in future.
Introduction
Bacterial diseases of plants are usually very difficult to control and toxicity of pesticides is now well known. Application of antibiotics decreases the citrus canker disease but these antibiotics are expensive [1]. Nevertheless, important agricultural crops suffer from at least one bacterial disease, and bacterial disease is the main cause of yield losses for some crops [2]. So, effort is necessary to reduce the losses caused by bacterial diseases.
Citrus canker caused by X. axonopodis is one of the most devastating economically important bacterial diseases of several citrus species. The disease occurs in citrus growing countries including Bangladesh and is endemic throughout Asia and many countries bordering the Indian Ocean [3].
In general, grapes, limes, sour oranges, lemons and oranges are highly susceptible. Infection causes lesions on the leaves, stems, and fruit of citrus trees, including lime, oranges, and grapefruit. Secondary rotting organisms invade lesions, causing fruit to rot. The primary symptoms of Citrus canker are leaf and twig-spotting. It is a disease of tropical and subtropical regions but it can occur and may become established in temperate in the absence of adequate control measures. In this regard, this experiment was taken to identify and test the antibiotic sensitivity pattern of the casual organisms of citrus canker disease of citrus plants available in Sylhet region of Bangladesh.
Methods
Collection and processing of samples: A total of 9 diseased plant samples were collected from different nurseries located at airport road, Chowhatta and Shahjalal University of Science and Technology of Sylhet city, Bangladesh. The minimum distance among the nurseries was approximately 2 kilometers. Leaves and fruits of orange, olive and lemon were collected in sterile packets. At each time of collection, precaution was taken to minimize cross contamination of samples. 1 ml of fruits rinsed water and fruit juices sample was added to a test-tube containing 9 ml of sterile water and thoroughly mixed to get a 10-1 dilution of the water sample. 1 ml of 10-1 dilution was transferred again to another 9 ml of sterile water in another test-tube and thoroughly mixed to get a 10-2 dilution. In such way serial dilution of water samples were made up to 10-4.
Isolation, purification, and preservation of the isolates: Isolation of X. axonopodis pv. Citri was done in NA and YDC media [4]. YDC was prepared by dissolving yeast extract 5.0 g, CaCO3 10.0 g, D-glucose 10.0 g and Bacto agar 8.5.0 g into 500 ml of distilled water. It was gently heated until dissolved. The pH was adjusted to 6.4-6.8 using 0.1N NaOH or 0.1M HCl as needed. It sterilized by autoclaving at 1210C, 15 psi for 15 minutes. Suspected colonies were transferred onto YDC medium and Incubated at 28 °C for 2-3 days.
Preparation and preservation of pure culture plate: Suspected single colonies of Xanthomonas axonopodis were taken from YDC plate respectively by sterile loop and inoculated on the NA medium. The plates were then incubated at 28oC for 24 hours after incubation of the pure culture; isolates were stored in sterilized 50% glycerol and were used as stock culture. They were stored at 4◦C.
Identification of the isolates
Colony morphology: The X. axonopodis pv. citri colonies grown on YDC were yellow and mucoid. They were yellow, convex and mucoid and differed in colony size (1 - 3 mm).
Gram staining: Gram’s staining was performed to determine the size, shape, arrangement and Gram reaction of the isolates.
Growth at different temperature: Single colony of X. axonopodis were taken from KB and YDC plate, respectively by sterile loop and inoculated on the NA medium. The plates were then incubated at different temperature.
Growth at 4% NaCl: Single colony of X. axonopodis were taken from KB and YDC plate respectively by sterile loop and inoculated on the NA medium. The plates were then incubated at 280C for 24 h.
Fluorescence under UV: Colonies of X. axonopodis on King’s B non-fluorescent under UV light at 366 nm after 48 h were observed. This allows the distinction from fluorescent Pseudomonads.
Biochemical tests: Biochemical tests (Oxidative-Fermentative Test, Nitrate Reduction Test, Citrate Utilization Test, Urease Test, Sucrose fermentation test, TSI (Triple Sugar Iodine) test, Mannitol fermentation test, Gelatine Hydrolysis) were done.
Antibiotic susceptibility test
Susceptibility of isolates to different antibacterial agents was determined in-vitro by employing a modified disk diffusion test of the Kirby-Bauer method [6]. The procedure involved measuring the diameter of the zone of inhibition that results from diffusion of the agent into the medium surrounding the disc. Antibiotic disk streptomycin (S) 10 µg, Gentamycin (GEN) 10 µg, Chloramphenicol (C) 30 µg, Ciprofloxacin(CIP) 5 µg , Bacitracin (B) 10 µg, (Becton Dickinson & co. USA) were used for the test.
Susceptibility test was performed for identified X. axonopodis strains by using Mueller-Hinton agar (CM337-OXOID) and 5 different antibiotic discs. This medium was prepared according to manufacturer’s instructions and sterilized by autoclaving at 121°C for 15 min, 15 lb/ inch2. The medium was then plated on sterile petri dishes and allowed to solidify [5]. 5 ml nutrient broth was dispensed in screw-capped test tubes and sterilized for inoculums preparation. After autoclaving, identified Xanthomonas axonopodis were inoculated in the sterilized test tubes containing the medium and placed in an incubator for 24 hours at 28°C. To maintain the same bacterial density during susceptibility testing, OD600 was measured by using Spectrophotometer. For each susceptibility test, 250 µl of microbial inoculums from cultured nutrient broth was spread on the surface of Mueller-Hinton agar. Then the antibiotic discs were applied on the inoculated plates with the help of needle and incubated at 28ºC for 20 hours in an inverted position. The results were recorded as zone of inhibition from the standard table. Antimicrobial agents and their disc concentrations are given at table 3. The diameters of the clear zone were measured. Xanthomonas axonopodi were recorded as sensitive, intermediate, and resistant to specific antibiotic depending on the areas of inhibition zone diameter they produced around them.
Herbal sensitivity studies
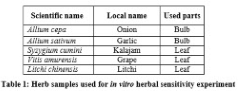
A total of 5 plants (table 4) extracts were used for the herbal sensitivity experiments. Selected parts of plant are washed and then rinsed with sterilized distilled water. Bulb and leaf extracts (table 4) were collected in a falcon tube and centrifuged at 4000 rpm for few minutes. A suspension of freshly cultured experimental bacteria was prepared for each strain separately. The antimicrobial activity of the plant extracts was evaluated using Agar Well Diffusion method and 0.1 ml of diluted inoculums of the isolated strains was swabbed on the nutrient agar plates. Wells of 5 mm diameter were punched into the agar plates with the help of sterilized cork borer (5 mm).
Using a micropipette, 50 µl of the plant extracts were added to the wells made in the plate. Herbal extracts Inhibitory response was recorded according to the normal growth response of the bacteria after incubation at 28◦C for 24 hours. Normal growth was recognized as resistance to extracts and clear zone was recognized as sensitive one. Zone of inhibition was measured by millimeter. Plant extracts and their disc concentrations are given at table 1.
Results
Isolation and identification of bacteria
The collected samples from the plants showing symptoms of citrus canker were inoculated into NB, then incubated at 28◦C for overnight and followed by inoculation on YDC media plates. The X. axonopodis colonies grown on YDC were yellow and mucoid.
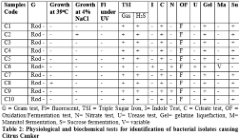
Gram staining: From 10 isolates, a total of 9 bacteria were found as gram negative rod shaped bacteria.
Growth at different temperature: The optimum temperature for X. axonopodis was found 28ºC- 30ºC and no growth was observed for 9 isolates at 39ºC.
Growth at 4% salt concentration: Growth of 9 isolates was not observed.
Fluorescence under UV: Colonies of Xanthomonas axonopodis were non fluorescent under UV.
Biochemical tests
For biochemical characterization, a series of biochemical tests were performed with the suspected gram negative bacteria. All biochemical tests’ results are given in table 2. After analyzing the results for all bacterial isolates, it was confirmed that 9 isolates are X. axonopodis. Identification was done by morphological, physiological and biochemical tests according to the EPPO/CABI standards diagnostic protocol [6]. The bacterium was found negative for nitrate reduction, indole production and for methyl red test.
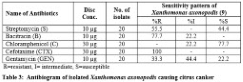
Antibiotic susceptibility test: Table 3 indicates the sensitivity pattern against commonly used antibiotics in citrus canker. All the isolates offered high degree of resistance against the commonly used antibiotics.
By comparing the zone created by the isolates with the standard zone of inhibition, all isolates were found 100% resistant to a cefotaxime (CTX). Chloramphenicol was found as the most effective among the tested antibiotics. X. axonopodis were found resistant (R) for GEN-33.3%, B-77.7%, S-55.5%, CTX-100%, C-0%; intermediate (I) were GEN-44.4%, B-22.2%, S-0%, CTX-0%, C-22.2% & susceptible (S) were GEN-22.2%, B-0%, S-44.4%, CTX-0%, C-77.7%.
Herbal sensitivity test: Antibacterial activity of aqueous extracts of five plants presented in table 4. Highly significant antibacterial activity was observed in A. sativum and S. cumini, respectively against the tested pathogens.
Data & Figures
Discussion
Citrus canker is an alarming hazardous threat to citrus fruits and for the citrus producing economy. Proper understanding of the pathogenic specialization of this pathogen is necessary. The study was conducted to determine the presence of X. axonopodis in diseased plant samples with the symptoms of citrus canker. The study was also aimed to find out sensitivity of isolated X. axonopodis to various antimicrobial agents widely used for controlling these diseases and to some plant extracts. Because copper-based bactericides are a standard control measure for citrus canker world-wide [7,8] and after long-term use resistance to copper in Xanthomonas populations was claimed [9]. Bacteria were isolated from plant samples and identified using cultural, physiological and biochemical tests. X. axonopodis was a gram negative bacterium with polar flagella [10]. The bacterium was rod-shaped and had a single polar flagellum. Growth was aerobic. The optimum temperature range for growth was 28ºC to 30°C. Bacterial cells were positive for hydrolysis of starch, casein, liquefaction of gelatine, reducing substance from sucrose, and H2S. It was found as the most potent antibiotic against the isolated organisms. Multidrug resistance was seen in case of some isolates of X. axonopodis pv. citri. Such incidence of multidrug resistance may presumably be due to indiscriminate use of drugs, lack of proper knowledge, negligence toward disease at the present time, which may eventually supersede the drug sensitive micro-organisms from antibiotic saturated environments.
Since all citrus cultivars are susceptible to canker, prevention, quarantines, control and eradication programs with drugs are the only tools available to control the disease, but come at a high annual cost for producers [11]. Considering the above facts, herbal sensitivity test was done for suggesting biological control agents. In recent years herbs became effective means of treatment for recovery of various diseases for the prevention and control the microbial diseases received increasing attention as alternative treatment of chemotherapeutics. In previous works, two hundred and eight diffusates from various plants such as forest trees, herbs, shrubs, fruit trees, spices, vegetables, food legumes, fodder, oil seed, fiber crops, cereals and ornamentals were evaluated through agar diffusion assay to determine their inhibitory action against X. axonopodis [12]. Besides this, more work had been done for antimicrobial activity of plant extracts against different Xanthmonas plant pathogens and leaf extract of Camellia sinensis were found most effective [13]. The present study concealed with locally available and commonly plant species that have an important role in inhibition of bacterial growth [14]. In the present study 5 plant samples were used for antimicrobial study and S. cumini, A. sativum were proved highly effective. The herbal sensitivity test has paved the way the viable introduction of plants for the treatment of disease causing microorganism in cheaper cost and eco-friendly way.
Most antibiotics with great effectiveness in-vitro do not necessarily show satisfactory effects in the real field. In this study all the isolates of X. axonopodis were found resistant to at least one or more of the commonly used antibiotics tested. Few isolates were found resistant for multiple antibiotics. Emphasis must be placed on the development of effective bactericides and their proper use with knowledge of the appropriate dosage. It will be more beneficial to put emphasis on biological control of citrus canker. Antibacterial activity was observed in A. sativum and S. cumini, respectively against the tested pathogens. The methanol and aqueous extracts of leaves of six different medicinal plants, Acalypha indica, Aerva lanata, Phyllanthus amarus, Phyllanthus emblica, Cassia auriculata and Caesalpinia pulcherrima, were used for the investigation of antibacterial studies of X. axonopodis. Garlic (A. sativum) has an antimicrobial activity which secretes sulfoxide, sulfatedter penoids, saponins compounds [15,16]. Onion (Allium cepa) secretes sulfoxide which is also effective against bacteria [17].
From this study it can be concluded that bulb extracts of A. sativum and leaf extract of S. cumini could be an effective measure for controlling plant diseases like citrus canker.
References
- Sahi ST, Ghazanfar MU, Afzal M, Rashed A, Habib A. Incidence of citrus canker disease caused by Xanthomonas campestris pv. citri (Hasse) dows on Kinnow (Citrus reticulata) and its chemotherapy. PAKISTAN JOURNAL OF BOTANY, (2007); 39(4): 1319.
- Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular plant pathology, (2012); 13(6): 614-629.
- Pruvost O, Boher B, Brocherieux C, Nicole M, Chiroleu F. Survival of Xanthomonas axonopodis pv. citri in leaf lesions under tropical environmental conditions and simulated splash dispersal of inoculum. Phytopathology, (2002); 92(4): 336-346.
- Scortichini M, Marchesi U, Di Prospero P. Genetic diversity of Xanthomonas arboricola pv. juglandis (synonyms: X. campestris pv. juglandis; X. juglandis pv. juglandis) strains from different geographical areas shown by repetitive polymerase chain reaction genomic fingerprinting. Journal of Phytopathology, (2001); 149(6): 325-332.
- Bauer A, Kirby W, Sherris JC, turck, Turck M. Antibiotic susceptibility testing by a standardized single disk method. American journal of clinical pathology, (1966); 45(4): 493.
- Ordax M, Biosca E, Wimalajeewa S, López M, Marco‐Noales E. Survival of Erwinia amylovora in mature apple fruit calyces through the viable but nonculturable (VBNC) state. Journal of applied microbiology, (2009); 107(1): 106-116.
- Koizumi M. Citrus canker: The world situation. Citrus Canker: An International Perspective LW Timmer, ed University of Florida, Lake Alfred, (1985); 2-7.
- Leite Jr R, Mohan S. Integrated management of the citrus bacterial canker disease caused by Xanthomonas campestris pv. citri in the State of Paraná, Brazil. Crop Protection, (1990); 9(1): 3-7.
- Rinaldi D, Leite Jr R. Adaptation of Xanthomonas axonopodis pv. citri population to the presence of copper compounds in nature; 2000. pp. 1064.
- Das A. Citrus canker-A review. Journal of Applied Horticulture, (2003); 5(1): 52-60.
- Beheshti B, Sharifi-Sirchi G, Mansouri M, Hosseinipour A, Schlaich N. Resistance to citrus canker in Key/Mexican Lime Induced by β-Aminobutyric Acid and green tea. American Journal of Agricultural and Biological Science, (2011).
- Akhtar MA, Rahber-Bhatti M, Aslam M. Antibacterial activity of plant diffusate against Xanthomonas campestris pv. citri. International Journal of Pest Management, (1997); 43(2): 149-153.
- Satish S, Raveesha K, Janardhana G. Antibacterial activity of plant extracts on phytopathogenic Xanthomonas campestris pathovars. Letters in Applied Microbiology, (1999); 28(2): 145-147.
- Mosch J, Zeller W, Rieck M, Ullrich W. Further studies on plant extracts with a resistance induction effect against Erwinia amylovora; 1995. pp. 361-366.
- Naganawa R, Iwata N, Ishikawa K, Fukuda H, Fujino T, et al. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Applied and Environmental Microbiology, (1996); 62(11): 4238-4242.
- San-Blas G, Marino L, San-Blas F, Apitz-Castro R. Effect of ajoene on dimorphism of Paracoccidioides brasiliensis. Journal of medical and veterinary mycology, (1993); 31(2): 133-141.
- Vohora S, Rizwan M, Khan J. Medicinal uses of common Indian vegetables. Planta medica, (1973); 23(4): 381-39.