![]()
A cost effective preparative thin layer chromatography cleanup method for high performance liquid chromatography analysis of aflatoxins B1, B2 and G2
Naveed Ahmed, Sohail Hassan Khan*, Muhammad Ashraf Anjum, Abdul Rehman
Adv. life sci., vol. 2, no. 1, pp. 1-4, November 2014
*Corresponding Author: Sohail Hassan Khan (Email: sohailhassan64@gmail.com)
Author Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
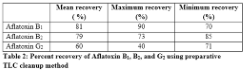
Aflatoxins are the by-products of fungal metabolism and common contaminants in feed. To keep their level below permissible limits, various assays have been developed. Currently, High Performance Liquid Chromatography (HPLC) with fluorescence detection of toxins is most widely used method but the cleanup methods requiring specific gadgets have increased the cost of the assay. Thin Layer Chromatography (TLC) is effective and economical but it only gives semi quantitative determination for aflatoxin. This study explores the preparative potential of TLC as cleanup method for HPLC analysis of toxin. Standard aflatoxins solutions containing 10 ng of B1, 2.5 ng of B2 and 2.5 ng of G2 were spotted on silica plates and then extracted using chloroform and acetone (4:1). The extracted toxins were resolved and quantified on HPLC using fluorescent detection. The results showed 81, 79 and 60% mean recovery of aflatoxin B1, B2 and G2, respectively. This method was proved equivocally comparable to other methods of aflatoxins cleanup and thus can be used as an alternative cost effective cleanup method.
Key words: Aflatoxins, Thin Layer Chromatography, High Performance Liquid Chromatography
Introduction
Aflatoxins are naturally produced secondary metabolites by some molds species, which contaminate the agricultural products in the field and/or while post-harvest duration. [1]. Aflatoxins are chemically difuranocoumarin compounds and include B1, B2, B2a G1, G2, G2a, M1 and M2. These are mainly produced by various Aspregilluss section such as A. flavus, A. parasiticus and A. nomius [2]. These toxins produce various pathological effects in humans, animals and birds including hepatotoxicity, teratogenesis and mutagenesis [3]. In poultry, these are associated with necrotic lesions in oral mucosa and intestinal tract, immune suppression and production losses [4]. Therefore, the level of aflatoxins is strictly monitored in food and feedstuff to keep it below the permissible limits. US Department of Agriculture and US Food and Drug Administration (FDA) have established an actionable level of 20 ppb of aflatoxins in animal feeds other than corn and cottonseed meal [5].
The qualitative and quantitative detection of aflatoxins has become possible by variety of techniques. These techniques are based on immunochemical, electrochemical and optical properties of aflatoxins such as enzyme linked immune-sorbent assay (ELISA), immune sensors, dipsticks, strip-tests, thin layer chromatography (TLC), high performance liquid chromatography (HPLC) with or without fluorescence detection and liquid chromatography tandem mass spectroscopy (LC/MS) [6,7].
Some newer techniques for this purpose are capillary electrophoresis, adsorptive stripping voltammetry, electrochemical transduction, and amperometric detection [8-10]. Each of these techniques has its merits and demerits in terms of efficiency, cost, time and expertise required.
HPLC coupled with fluorescence detection is one of the most sensitive and accurate method for aflatoxin determination. Principally, the aflatoxins are extracted from sample matrix through a polar solvent or a mixture of solvents, once extracted the aflatoxins are cleaned up through variety of methods to remove any impurities causing hindrance in the chromatogram interpretation. The cleanup requires solid phase extraction columns of silica or florisil and matrix solid phase extraction or immuno affinity columns, thereby increasing the cost of the assay [11].
TLC is simple, widely used, low cost and semi quantitative method of aflatoxin determination [12]. Using variety of solvent systems for extraction and cleanup, this method is applicable for different kinds of sample matrixes. TLC has both analytical and preparative potential for aflatoxin determination. The objective of this study is to explore the preparatory potential of TLC as a replacement of other cleanup methods for quantification of aflatoxins through HPLC with fluorescence detection.
Methods
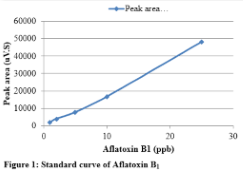
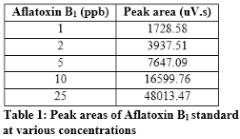
Standard solution containing 2 ppm of aflatoxin B1, 0.5 ppm of aflatoxin B2 and 0.5 ppm of aflatoxin G2 was diluted to get standard solutions of 1, 2, 5, 10 and 25 ppb of B1; 0.25, 0.5, 1.25, 2.5 and 6.25 ppb of B2 and 0.25, 0.5, 1.25, 2.5 and 6.25 ppb of G2. Perkin Elmer series 200 HPLC systems with TotalChrom Workstation was used for data analysis. The standards were run in mobile phase containing 60% water, 20% methanol and 20% acetonitrile while keeping the flow rate of 1 mL/min. The C-18 column was used as stationary phase to resolve each toxin. Fluorescence detector was used at excitation wavelength of 360 nm and emission wavelength of 440 nm. The standard curve for each toxin was drawn by taking the peak area and plotting it against known amount. Peak areas of aflatoxin B1standard at various concentrations are given in Table 1. The standard curve of B1 is shown in Figure1. Chromatogram of standard solution of aflatoxin B1, B2 and G2, resolved using mobile phase containing 60% water, 20% methanol, and 20% acetonitrile is given in Figure 2.
A volume of 80 uL of aflatoxin standard containing 10ng of B1, 2.5 ng of B2 and 2.5 ng of G2 was dissolved in acetonitrile and spotted on TLC silica coated plates. The plates were run in mobile phase containing 10% acetone and 90% chloroform. After the development of plates, they were visualized under UV light (254 nm) and the portion of plates containing spots were excised. The excised portions of plates were dipped in chloroform and acetone (4:1) for 15 minutes. The excised portion were re-examined under UV light to confirm the elution. The chloroform and acetone were evaporated to dryness and the aflatoxins were re-dissolved in 1 mL of acetonitrile and filtered through 0.45 um filter to separate any silica particles left. The filtrate was examined through HPLC using florescence detector and setting the conditions similar as for standards. The chromatogram of extracted standard solution by above mentioned method is shown in Figure 3. The amount of eluted aflatoxins was determined using calibration curve of aflatoxins standards containing known amounts of each aflatoxin B1, B2 and G2 and their percentage recovery was established.
Results
Table 2 shows the percent recovery of standards containing 10 ng of aflatoxin B1, 2.5 ng of B2 and 2.5 ng of G2. The percentage recovery of aflatoxins from this method was comparable to other cleanup methods, such as immune affinity columns and solid phase extraction system, assuming complete extraction of aflatoxins by TLC cleanup method used. Since extracted potential of various TLC methods is well established, therefore, while using this method, percentage extraction potential of TLC from different matrixes should be considered for final calculations.
Data and Tables
Discussion
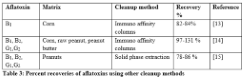
Quantification potential of TLC is limited but the cost of sample preparation is economical as compared to other methods. Various standard TLC methods for aflatoxin determination can be combined with HPLC exploiting quantitative potential of HPLC. The visual observation of toxins under UV light before HPLC analysis enhances the validity of this method. Good cleanup of samples before spotting on TLC plate will greatly reduce the noise between peaks. The results of this study show that preparative TLC can be used as an alternate precise method to other cleanup methods for aflatoxin analysis on HPLC thus reducing the cost of assay. The extraction potential of immune affinity and solid phase extraction system from various matrices is shown in Table 3. Brera et al. extracted 82-84% of aflatoxin B1 from corn, whereas, Trucksess et al. demonstrated 97-131% recovery of aflatoxin B1, B2, G1 and G2 using immune affinity columns [13,14]. Recovery of aflatoxins B1, B2, G1 and G2 was 78-86% in peanuts when solid phase extraction system was employed [15].
The TLC cleanup method used in this study can be an alternative to immune affinity and solid phase extraction cleanup for quantification of aflatoxins on HPLC system using fluorescent detection. It can be used as in-house cost effective protocol in combination with various TLC extraction systems. For accurate quantification of aflatoxins from various matrixes, extraction potential of TLC extraction procedure should be established for that particular matrix and final calculations should be adjusted accordingly.
References
- Cotty PJ, Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. International Journal of Food Microbiology, (2007); 119(1–2): 109-115.
- Abrar M, Anjum FM, Butt MS, Pasha I, Randhawa MA, et al. Aflatoxins: Biosynthesis, Occurrence, Toxicity, and Remedies. Critical Reviews in Food Science and Nutrition, (2012); 53(8): 862-874.
- Godfrey SB, David K, Lubega A, Jasper O-O, William WA, et al. (2013) Review of the Biological and Health Effects of Aflatoxins on Body Organs and Body Systems.
- Rawal S, Kim JE, Coulombe Jr R. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Research in Veterinary Science, (2010); 89(3): 325-331.
- FDA. Action Levels for Aflatoxins in Animal Feeds. FDA compliance policy guide, (1989).
- Do J, Choi D-K. Aflatoxins: Detection, toxicity, and biosynthesis. Biotechnology and Bioprocess Engineering, (2007); 12(6): 585-593.
- López Grío SJ, Garrido Frenich A, Martínez Vidal JL, Romero-González R. Determination of aflatoxins B1, B2, G1, G2 and ochratoxin A in animal feed by ultra high-performance liquid chromatography–tandem mass spectrometry. Journal of Separation Science, (2010); 33(4-5): 502-508.
- Paniel N, Radoi A, Marty J-L. Development of an Electrochemical Biosensor for the Detection of Aflatoxin M1 in Milk. Sensors, (2010); 10(10): 9439-9448.
- Hajian R, Ensafi AA. Determination of aflatoxins B1 and B2 by adsorptive cathodic stripping voltammetry in groundnut. Food Chemistry, (2009); 115(3): 1034-1037.
- Maragos CM (2000) Measurement of Aflatoxins Using Capillary Electrophoresis. pp. 51-58.
- Buttinger G. Aflatoxin measurements: how HPLC methods have evolved over the last 20 years? Food Additives & Contaminants: Part A, (2010); 27(9): 1266-1272.
- Betina V. Thin-layer chromatography of mycotoxins. J Chromatogr, (1985); 334(3): 211-276.
- Brera C, Debegnach F, Minardi V, Pannunzi E, Santis BD, et al. Immunoaffinity column cleanup with liquid chromatography for determination of aflatoxin B1 in corn samples: interlaboratory study. Journal of AOAC International, (2007); 90(3): 765-772.
- Trucksess MW, Stack ME, Nesheim S, Page SW, Albert RH, et al. Immunoaffinity column coupled with solution fluorometry or liquid chromatography postcolumn derivatization for determination of aflatoxins in corn, peanuts, and peanut butter: collaborative study. Journal-Association of Official Analytical Chemists, (1990); 74(1): 81-88.
- Blesa J, Soriano J, Molto J, Marın R, Manes J. Determination of aflatoxins in peanuts by matrix solid-phase dispersion and liquid chromatography. Journal of Chromatography A, (2003); 1011(1): 49-54.