![]()
Study of drug mediated effects in mice: Histology based findings
Mehwish Altaf1, Sidra Hasnain2*, Muhammad RaziUllah Khan2, Muhammad Wasim Khan3
Adv. life sci., vol. 2, no. 3, pp. 119-124, May 2015
*- Corresponding Author: Sidra Hasnain (Email: sidrahasnain346@gmail.com)
Author Affiliations[Date Received: 27/02/2015; Date Revised: 30/04/2015; Date Published Online: 25/05/2015]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Drugs induce numerous kinds of pharmacological effects in different body organs. For protecting organs from damage and destruction from drugs, the study of such effects is extremely important. Rapidly accumulating experimental data has opened new horizons for a comprehensive re-conceptualization of chemical modulated changes in histological features of body tissues.
Methods: In this study we studied dextromethorphan, ethanol, methanol and midazolam induced changes in histological specimen from different organs in post-treated mice.
Results: No pathological changes were observed in heart, liver and kidney by administering dextromethorphan at a dose of 61 mg/kg. While, ethanol causes pathological changes in heart, liver and kidney tissue at high dose i.e. 2000 mg/kg. Gross Pathological changes were observed in heart, liver and kidney by giving midazolam at a dose of 200 mg/kg.
Conclusion: Dextromethorphan showed lesser side effects and is less toxic as compared with other drugs, such as, ethanol, methanol and midazolam. Lesser toxic effects were observed when drugs were administered alone, but in combination, these drugs produced higher toxic effects.
Key wrods: Dextromethorphan, Ethanol, Methanol, Midazolam, Pharmacological effects, Mice, Histopathology
Introduction
Data obtained through preclinical and clinical studies has considerably improved our understanding of drug mediated effects on histological features of different organs. Midazolam is a short-acting depressant of the central nervous system (CNS) [1]. It has sedative, anxiolytic, amnesic and hypnotic properties. Its activity is due to the reversible interaction of receptor gamma amino butyric acid (GABA) in the CNS. GABA is the major neurotransmitter inhibitor in the central nervous system (CNS) [2]. Its action is reversed by flumazenil. Benzodiazepines produce various effects including relaxation of skeletal muscles and even sleep, by increasing the activity of GABA. This activity is increased by increasing the opening of chloride channels. Dextromethorphan is one of the most effective cough suppressant frequently used in cough syrups available in the market. It is an opioid derivative of morphine and is the dextro-isomer of levorphanol obtained by methylation. Midazolam and diazepam are sedating agents. Midazolam is administered through intramuscular route and diazepam through oral route. Both are given to preoperative sedative patients who have taken anesthesia. It was observed that midazolam is less toxic at low dose when administered to animals [3]. Higher dose produced serious side effects like increased weight of liver due to increase in ALP. Furthermore, midazolam is safe for biological use as it has no embryotoxic, teratogenic and mutagenic effects. Overdose of chlorphenaramine and dextromethorphan were checked to find which one is responsible for serotonin toxicity in patients. One hundred and fifty review articles were found which shows the poisoning effect of dextromethorphan and chlorphenaramine out of which 23 were of dextromethorphan in which 18 were excluded because of no effect of serotonin and chlorphenaramine toxicity. Remaining 16 shows the serotonin toxicity of chlorphenaramine and dextromethorphan because of overdose of these drugs. Thus, it was discussed [4] that chlorphenaramine is a potent drug inhibiting reuptake of serotonin same in manner as dextromethorphan.
The objective of the present study was to assess the major toxic effects of ethanol, methanol, midazolam and dextromethorphan on different target organs (liver, kidney and heart).
Methods
Selection of animals
Healthy female albino mice, each weighing 25-30g (8 to 12 weeks old), were used for the present study. All females mice used in this study were nulliparous and non-pregnant according to OECD policy, 2008. The animals were divided in different groups each with 5 mice per cage and were housed in animal house of Faculty of Pharmacy, The University of Lahore. The animals were kept under standard laboratory conditions, such as, temperature (22 ± 3˚C), relative humidity (30%), and 12 hrs day/night cycles. LD50 = 1600 mg/kg, 75 mg/kg and 50 mg/kg were administered to rat and mouse at various doses on 13 days observation period.
Preparation of doses
Drugs were carefully administered using different concentrations and fixed volumes. The concentration of each drug dose was kept as high as 1 ml/100 g of body weight, however, in case of aqueous solution 2 ml/100 g of weight were considered. For other vehicle that is other than water the toxicological character of vehicle should be known. Doses were administered by oral gavage. Animals were fasted prior to dosing i.e. food was withheld for 3-4 hour, however, water was provided ad libitum. Animals were weighed during fasting period and doses were calculated accordingly (OECD, 2008).
Limit test at 2000 mg/kg
Initially, single test dose was administered to all the five animals in each group. Dose was increased up to four times if all the animals survived, whereas, death of at least 3 out of 5 animals was used as a criterion for the termination of limit test and switching to main test. LD50 should be more than 2000 mg/kg if 3 or more animals survived. LD50 varied in different subjects some may be killed at very low dose or some may be survived at higher dose more than LD50. So, more animals died at low dose but when dose was increased more than 2000 mg/kg, they survived.
Main test
After administration of first dose, 48 hrs interval was provided before administration of second dose. Duration, onset and severity of toxic signs were determined during the time interval between dosing. Initial dose given to the first animal was lower than the approximate value of LD50. If the animal survived then next dose was increased. If the first animal died then second animal received lower dose as compared to the previous one. A progression factor of 3.2 was used in dose response curve when slope of substance did not give any response otherwise it remained constant throughout testing. If slope was found less than 2, which means highly variable tolerance of animals, then progression factor dose was increased by giving consideration on a large dose scale i.e. 3.2 progression factors to start the test. Same was used in the case of test for the known substances, in which they had very high gradient slopes and dose of progression was chosen lesser than the default dose (OECD, 2008).
Study design
The present study was conducted by using a total of thirty five mice. Animals were divided into 7 groups; each group consisted of 5 mice. These animals were treated with single dose of dextromethorphan (61 mg/kg), ethanol (2000 mg/kg), methanol (2000 mg/kg), midazolam (200 mg/kg) and combined doses of dextromethorphan + ethanol (200 mg/kg + 2000 mg/kg), dextromethorphan + methanol (200 mg/kg + 2000 mg/kg) and dextromethorphan + midazolam (200 mg/kg + 200 mg/kg). Animals were dissected under light anesthesia and vital organs, such as; liver, heart, and kidney were removed. The organs were observed under compound microscope for gross pathological analysis.
Histopathological examination of heart, liver and kidney
Liver, heart and kidney of mice were removed. These organs were fixed in 10% formalin and eventually decalcified by using HCl, ethylenediamine tetra-acetic acid (EDTA), sodium tartrate and potassium sodium tartrate. Tissues were then implanted in paraffin and blemished with hematoxylin and eosin after cutting into 5 µm thick sections [5].
Results
Gross pathological
Gross pathological changes were observed in various organs like heart, liver and kidney by administering single and combined doses of drugs in female albino mice. Results are as following:
Control Cases
Treated Cases
Dextromethorphan:
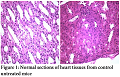
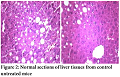
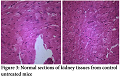
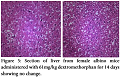
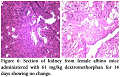
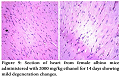
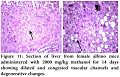
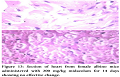
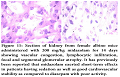
No pathological changes were observed in heart, liver and kidney by administering dextromethorphan at a dose of 61 mg/kg. Section of heart from female albino mice administered with 61 mg/kg dextromethorphan for 14 days showed no pathological change. No pathological changes during gross histological investigation were noticed in liver and kidney tissue as well. All the sample preparation and to-follow microscopic visualization was done right after sacrificing the mice.
Ethanol
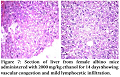
Pathological changes were observed in heart, liver and kidney by giving ethanol at a dose of 2000 mg/kg.
Methanol
Pathological changes were observed in heart, liver and kidney by giving methanol at a dose of 2000 mg/kg.
Midazolam
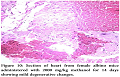
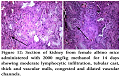
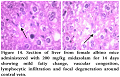
Gross Pathological changes were observed in heart, liver and kidney by giving midazolam at a dose of 200 mg/kg.
Discussion
Midazolam is a highly potent sedative drug. It has fast duration of action and cause sedation in short period of time at different levels such as endoscopic procedures, pre-operative sedation and general anesthesia. It has good local tolerability and highly stable hemodynamic activity [6]. A previous study also showed that midazolam dose dependently produced liver toxicity [3]. Gross pathological changes in heart, liver, and kidney was observed in our study when midazolam was administered alone or in combination with dextromethorphan. This is in line with the previous study which showed that administration of midazolam at various doses from lowest to highest caused increase in weight of liver in both male and female rats along with gross histopathological changes. Dextromethorphan produced fewer side effects but if given in large quantities, it produced dissociative effect as compared with other excessively used drugs [7]. High dose of ethanol is also known for increasing the levels of hepatic and renal parameters [8]. Present study demonstrated that dextromethorphan alone did not cause pathological changes in heart, liver, and kidney, until provided in combination of other drugs. A previous study demonstrated that high dose of dextromethorphan did not cause any pathological change in kidney, heart, liver, spleen and pancreas [9]. Dextromethorphan is absorbed by gastrointestinal tract and enters in the blood stream from where it passes into brain through blood brain barrier and even account for variety of behavioral issues [10].
Dextromethorphan showed lesser side effects and is less toxic as compared with other drugs, such as, ethanol, methanol and midazolam. Lesser toxic effects were observed when drugs were administered alone, however, when given in combination; these drugs produced higher toxic effects. Present study suggests that these drugs are relatively safer when administered alone at low doses as compared with combinational use of these drugs.
Future studies may provide a converge on a deeper and broader analysis of drug induced effects at cellular level. Furthermore, discovery of next generation of targets and imaging biomarkers may also be helpful in tailoring the therapy to the patient.
References
- Bolon M, Boulieu R, Flamens C, Paulus S, Bastien O. Sedation induced by midazolam in intensive care: pharmacologic and pharmacokinetic aspects. Annales Francaises D'anesthesie et de Reanimation, (2002); 21(6): 478-492.
- Orser B, McAdam L, Roder S, MacDonald J. General anaesthetics and their effects on GABA A receptor desensitization. Toxicology Letters, (1998); 100: 217-224.
- Schlappi B. Safety aspects of midazolam. British Journal of Clinical Pharmacology, (1983); 16(S1): 37S-41S.
- Monte AA, Chuang R, Bodmer M. Dextromethorphan, chlorphenamine and serotonin toxicity: case report and systematic literature review. British Journal of Clinical Pharmacology, (2010); 70(6): 794-798.
- Shabbir A, Shahzad M, Ali A, Zia-ur-Rehman M. Anti-arthritic activity of N′-[(2, 4-dihydroxyphenyl) methylidene]-2-(3, 4-dimethyl-5, 5-dioxidopyrazolo [4, 3-c][1, 2] benzothiazin-1 (4H)-yl) acetohydrazide. European Journal of Pharmacology, (2014); 738: 263-272.
- Suri CY. Evaluation of midazolam and diazepam for pre operative Sedation. Medical Journal Armed Forces India, (2014); 56: 287-292.
- Romanelli F, Smith KM. Dextromethorphan abuse: clinical effects and management. Pharmacy Today, (2009); 348-355.
- Sankaran M. Protective effect of Solanum nigrum fruit extract on the functional status of liver and kidney against ethanol induced toxicity. Journal of Biochemical Technology, (2012); 3(4): 339-343.
- Bar-Shai A, Starr A, Schwarz Y. Toxicity and safety of dextromethorphan inhalation in a mouse model prior its use in humans. European Respiratory Journal, (2011); 38(Suppl 55): p841.
- Boyer EW, Shannon M. The serotonin syndrome. New England Journal of Medicine, (2005); 352(11): 1112-1120.