![]()
Sero-prevalence of Human Cytomegalovirus among blood donors in Lahore, Pakistan
Chahat Batool Rizvi1, Ali Raza1, Maria Fareed Siddiqui*2, Rabail Alam1
Adv. life sci., vol. 2, no. 4, pp. 171-175, August 2015
*- Corresponding Author: Maria Fareed Siddiqui (Email: maria.pharmacist@gmail.com)
Authors' Affiliations
2- Center for Research in Molecular Medicine, University of Lahore, Lahore- Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Transfusion-transmitted cytomegalovirus (TT-CMV) infection can cause severe illness and even death among immunocompromised patients; therefore, the spread of CMV through blood products should be prevented. To our knowledge, no study has been carried out in Pakistan to determine the seroprevalence of CMV in general population as well as among blood donors. The goal of this study was to determine CMV seropositivity among blood donors at the blood bank of INMOL Hospital, Lahore, Pakistan.
Methods: A sero-epidemiological cross-sectional study was conducted. Sera from 91 blood donors were screened for CMV specific IgG antibodies by enzyme-linked immunosorbent assay (ELISA) based kit.
Results: The CMV-specific IgG antibodies were detected in 89 blood donors, which gave seroprevalence rate of 97.8%. The statistical analysis of results was done using pearson chi-square test and appeared non-significant with values 0.625 and 0.705 for different age groups and blood groups of donors.
Conclusion: Because of high seroprevalence in this study area, an adequate supply of CMV seronegative blood is difficult to maintain. Therefore, we propose that the future strategies for the prevention of post-transfusion CMV infection in recipients should include the transfusion of leukoreduced blood products. Further a prospective study with much greater population can be done to identify major causative risk factors for such highest prevalence rate.
Keywords: Human Cytomegalovirus, Blood donors, Sero-prevalence, Leukoreduced blood, Post transfusion CMV infection
Introduction
Human cytomegalovirus (HCMV) is a DNA virus that belongs to beta sub-group of the herpesvirus family. It is also known as β human herpesvirus type 5. HCMV is a ubiquitous pathogen that causes infection in 40%-100% of adult population worldwide, mainly depending on socioeconomic status [1]. CMV is transmissible through transfusion of blood and blood products. Other routes of transmission are contact with infected body secretions, sexual contact, breast feeding, placental transfer, solid organ or hematopoietic stem cell transplantation [2].
Like other herpesviruses, after primary infection with HCMV, the virus develops a life-long latent infection within the host. Latent infection is characterized by a state, where the viral genome is sustained within the cell with limited viral gene expression and no viral progeny is produced [3]. Under certain circumstances, the virus may be reactivated from this latency to generate new infectious viral particles that can be transmitted to other susceptible hosts [4]. It is suggested that individuals with latent infection, harbor HCMV genomes in CD34+ hematopoietic stem cells, CD33+ granulocyte-macrophage progenitor cells and CD14+ monocytes. However, cells other than those mentioned may also harbor latent virus [5, 6].
HCMV infection shows low pathogenicity in healthy immunocompetent individuals and is usually asymptomatic or only causes mild symptoms, which may manifests as mononucleosis-like syndrome. Whereas, in immunologically immature and immunocompromised individuals, such as preterm low-birth weight infants (<1200 g), transplant patients (organ and bone marrow transplantation), AIDS patients and patients receiving extensive chemotherapy or other immunosuppressing therapy, infection with CMV is associated with high morbidity and mortality. In these individuals, CMV infection can result in leukopenia, malaise, encephalitis, hepatitis, pneumonia, gastroenteritis, retinitis and even death [2, 7].
Primary infection, reactivation, and reinfection (i.e. co- infection with a different strain) are the types of active HCMV infections that can occur in immunosuppressed individuals [8]. The most common serologic test used to identify CMV infection is the enzyme-linked immunosorbent assay (ELISA). The presence of CMV specific IgG antibody without specific IgM represents past exposure to CMV, whereas the presence of both CMV specific IgM and IgG usually indicates active infection [9, 10].
Transfusion-transmitted Cytomegalovirus (TT-CMV) can lead to primary infection in CMV-seronegative recipients or reinfection by a new strain in seropositive transfusion recipients. However, active infection can alternatively occur if transfusion stimulates reactivation of latent endogenous virus in seropositive recipients [10]. In CMV seropositive immunocompromised transfusion recipients, viral reactivation rather than a reinfection accounts for most of the active infections.
TT-CMV infection can lead to serious or even fatal clinical manifestations in high-risk transfusion recipients. The patient populations at high risk for CMV disease include CMV-seronegative patients in the following categories: pregnant women and their neonates, preterm low birth weight infants (<1200g), bone marrow and solid organ transplant recipients, AIDS patients, and other extremely immunosuppressed individuals, so these group of patients require CMV free blood or components [7, 11].
The most commonly used methods to reduce the risk of TT-CMV infection include transfusion of CMV-seronegative blood units and/or transfusion of pre-storage leukoreduced blood products. Leukoreduction decreases the risk of TT-CMV because latently infected white blood cells are reduced by this process. In addition, it may reduce endogenous virus reactivation [12, 13].
As there is lack of effective treatment for this infection, prevention remains the most effective way to avoid TT-CMV infection, especially in immunocompromised individuals. In Pakistan, the seroprevalence of CMV among general population and blood donors has not yet been analyzed. Therefore, our basic aim is to estimate the seroprevalence of CMV among blood donors by detecting the presence of CMV specific IgG antibodies. This study will be helpful in developing proper strategies for reducing the risk of transfusion-transmitted CMV infections, especially in immunocompromised patients.
Methods
A seroepidemiological cross-sectional study was done on blood samples from donors presenting at the blood bank of Institute of Nuclear Medicine & Oncology (INMOL), Lahore, from April to July 2013. The study population was comprised of 91 blood donors randomly selected from donors attending blood bank and considered fit to donate blood. All donors were male with an age of 18-49 years. The blood donors included in this prevalence study were divided into two groups: young adults with age ranging from 18-25 and older adults with age >25. The two seronegative donors found in this study were 22 years old and thus they were from “young adults” group. Each donor was asked and aided to fill the structured questionnaire to provide information regarding age, sex, occupation, education, marital status and history of blood donation. Ethical approval was obtained from the institution’s ethics committee and consent form was filled by each donor. Blood sampling was done in Blood Bank at INMOL.
The blood sample of 5 ml was collected from each donor after obtaining written consent. The serum of samples was separated by centrifugation at 3000 rpm for 10 minutes and then stored at -40oC until use. Sera were tested for CMV-specific IgG antibodies by enzyme-linked immunosorbent assay (ELISA), using Human CMV IgG ELISA kit (REF 51203; Human GmbH, 65205 Wiesbaden, Germany), in accordance with the manufacturer's instructions. Samples positive for anti-CMV IgG indicate the presence of a past or recent infection. Positive and negative standard sera, provided with the kit, were included in each assay.
The absorbance of controls and specimen was measured at 450nm by using ELISA microplate readers. Results for patient samples were obtained by comparing the obtained absorbance values to a calculated cut-off value 0.543. Samples with absorbance greater or equal to 0.543 were considered as positive for CMV IgG, while samples with absorbance below the cut-off as negative results. The seroprevalence of CMV-specific IgG antibodies was defined as the percentage of reactive samples. Statistical analysis was done by using Pearson chi-square test and significance or insignificance was found out.
Results
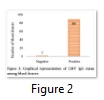
A total of 91 blood donors were screened for anti-CMV IgG. Their age ranged from 18 to 49 years with mean age of 25.87 ±6.8 years. All subjects were healthy male voluntary blood donors, who were distributed into groups of young adults (ages 18-25 years) and older adults (>25 years) (as shown in Fig. 1). CMV IgG was positive in 89 blood samples; only 2 samples were negative for CMV-specific IgG antibodies. Hence, the prevalence of positive CMV IgG among blood donors was 97.8%. Both of the two negative donors were of the age of 22 years (Fig. 2).
Tables and Figures
Discussion
In Pakistan, no study has been carried out to date to estimate the seroprevalence of CMV in blood donors. The seroprevalence of anti-CMV IgG among blood donors found in the present study was 97.8%, indicating highly prevalent CMV infection is in this study area. The pearson chi-square statistical analysis was done for different age groups and blood groups to identify any significance or insignificance. Non- significant values were appeared for both group with values 0.625 and 0.705. The high CMV seropositivity rate among blood donors observed in this study can be compared to the rates among blood donors within different regions of India i.e. 95%, 87.9% and 87%, from Delhi, Pune and Chennai, respectively [14-16]. High seroprevalence rate among blood donors was also reported in Ghana, Brazil and Nigeria as 93.2%, 96.4% and 95.8%, respectively [17-19]. The high seroprevalence observed in these countries is in contrast to those of western nations, which ranged from 38% to 75% [1]. The possible explanation for this distribution could be linked with socioeconomic status, as this infection is more prevalent in developing countries with low socioeconomic conditions, which is mainly associated to closeness of contacts within these populations [17].
Our results ended with only two negative donors and those were young males. The seronegative donors were expected from younger group as published by Hecker et al., that seroconversion is a lifelong event and associated with age [18]. Same results were also obtained by Ahmed et al., in 2006 [19].
CMV antibody positive blood donors are CMV carriers and contain latently infected cells in their blood leukocytes, chiefly peripheral blood monocytes, which can be reactivated after transfusion and thus may be infectious. One factor that may stimulate CMV reactivation is allogeneic reaction during blood transfusion [20]. The risk of TT-CMV infection after receiving seropositive blood products is estimated to be 0.4 to 12% [20]. It was suggested that donors who have anti-CMV IgM antibodies (CMV IgG +/-) are more infectious than IgG positive and IgM negative ones but this remained unconfirmed [11].
TT-CMV infection can cause adverse clinical complications among immunosuppressed patients; therefore, high CMV seropositivity rate among blood donors is of major concern for the safety of blood transfusion in high-risk recipients. Several published reports have shown that the risk of post-transfusion CMV infection in immune-compromised recipients can be reduced by providing blood from CMV-seronegative donors or by transfusing pre-storage leukoreduced blood components. The leukoreduced components are nearly equivalent to CMV seronegative donations if residual leukocytes are <5×106 per unit [12]. The reduction in the risk of TT-CMV infection was estimated to be 93.1% in CMV-seronegative components and 92.3% in leukoreduced components when compared with random blood components [13]. The existence of plasma viremia during the pre-seroconversion phase and the failure to attain sufficient reduction of leukocytes have been accounted for the residual risk of CMV in these blood products which is rarely encountered.
Due to high CMV seroprevalence in our donors, it is not practical and beneficial to screen blood donors routinely for CMV. Therefore, we propose that the future strategies to minimize the risk of TT-CMV infection in susceptible high-risk recipients should include the transfusion of pre-storage leukoreduced blood products.
More studies are needed, on a large scale, both in donor and patient populations in order to establish proper strategies for preventing and/or reducing the transmission of CMV through blood transfusion, especially in immunocompromised patients. The limitations of this study included the small sample size and no female volunteers presenting at the Blood Bank. Despite these limitations, the present study shows that donated blood at the Blood Bank of INMOL, Lahore, Pakistan have a high seropositivity for IgG antibodies to CMV.
Acknowledgement:
We sincerely appreciate the Staff of INMOL Blood Bank for their kind cooperation; and especially Mr. Tariq Bashir Sipra (ex-Deputy Chief Scientist at INMOL) and Mr. Azadar Rizvi (Principal Technologist at INMOL) for their excellent technical assistance and support.
References
- Krech U. Complement-fixing antibodies against cytomegalovirus in different parts of the world. Bulletin of the World Health Organization, (1973); 49(1): 103.
- Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clinical microbiology reviews, (2009); 22(1): 76-98.
- Baldick C, Marchini A, Patterson CE, Shenk T. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. Journal of Virology, (1997); 71(6): 4400-4408.
- Goodrum F, Caviness K, Zagallo P. Human cytomegalovirus persistence. Cellular Microbiology, (2012); 14(5): 644-655.
- Taylor-Wiedeman J, Sissons J, Borysiewicz LK, Sinclair J. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. The Journal of General Virology, (1991); 722059-2064.
- Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. Journal of General Virology, (2006); 87(7): 1763-1779.
- Steininger C. Clinical relevance of cytomegalovirus infection in patients with disorders of the immune system. Clinical Microbiology and Infection, (2007); 13(10): 953-963.
- Barbara J, Tegtmeier G. Cytomegalovirus and blood transfusion. Blood Reviews, (1987); 1(3): 207-211.
- Munro S, Hall B, Whybin L, Leader L, Robertson P, et al. Diagnosis of and screening for cytomegalovirus infection in pregnant women. Journal of Clinical Microbiology, (2005); 43(9): 4713-4718.
- Jahan M, Tabassum S, Aziz A, Ahmed M, Islam MN. Transfusion associated CMV infection: Transfusion strategies for high risk patients. Bangladesh Journal of Medical Microbiology, (2012); 4(2): 24-27.
- Badami K. The immunocompromised patient and transfusion. Postgraduate Medical Journal, (2001); 77(906): 230-234.
- Roback JD. CMV and blood transfusions. Reviews in Medical Virology, (2002); 12(4): 211-219.
- Vamvakas EC. Is white blood cell reduction equivalent to antibody screening in preventing transmission of cytomegalovirus by transfusion? A review of the literature and meta-analysis. Transfusion medicine reviews, (2005); 19(3): 181-199.
- Kothari A, Ramachandran V, Gupta P, Singh B, Talwar V. Seroprevalence of cytomegalovirus among voluntary blood donors in Delhi, India. Journal of Health, Population and Nutrition, (2002); 348-351.
- Chaudhari C, Bindra M. Seroprevalence of cytomegalovirus among voluntary blood donors. Medical Journal Armed Forces India, (2009); 65(3): 252-254.
- Sukrutha Gopal R, Radhika Chowdary D, Anil Kumar B. Seroprevalence of transfusion transmissible infections among healthy blood donors at KIMS blood bank. Journal of Medical and Scientific Research, (2014); 2(3): 137-139.
- de Jong MD, Galasso GJ, Gazzard B, Griffiths PD, Jabs DA, et al. Summary of the II international symposium on cytomegalovirus. Antiviral Research, (1998); 39(3): 141-162.
- Hecker M, Qiu D, Marquardt K, Bein G, Hackstein H. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sanguinis, (2004); 86(1): 41-44.
- Ahmed S, Al-Joudi F, Zaidah AW, Roshan T, Rapiaah M, et al. The prevalence of human cytomegalovirus seropositivity among blood donors at the Unit of Blood Transfusion Medicine, Hospital Universiti Sains Malaysia. The Southeast Asian Journal of Tropical Medicine and Public Health, (2006); 37(2): 294-296.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell, (1997); 91(1): 119-126.