![]()
Biosafety assessment of transgenic Bt cotton on model animals
Ahmad Ali Shahid1*, Sadia Bano1, Sana Khalid2, Tahir Rehman Samiullah1, Kamran Shahzad Bajwa1, Muhammad Azam Ali1
Adv. life sci., vol. 3, no. 3, pp. 97-108, May 2016
*- Corresponding Author: Ahmad Ali Shahid, (Email: ahmadali.shahid@gmail.com)
Authors' Affiliation
2- Department of Botany, Lahore College for Woman University, Lahore – Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: To know the effects of transgenic crops on soil microorganisms, animals and other expected hazards due to the introduction of GM crops into the environment is critical both scientifically and environmentally. The work was conducted to study the effect of insecticidal Bt protein on Rats and Earthworms.
Methods: For this purpose, animals like rat and soil organisms like Earthworm were selected. Rats were selected on the basis of its 95% homology on genomic, cellular and enzymatic level with human while earthworm were preferred on the basis of their direct contact with soil to evaluate the impact of Bt (Cry1AC) crop field soil on earthworm, secreted by root exudates of Bt cotton. Several physical, molecular, biochemical and histological analyses were performed on both Rats/Earthworms fed on standard diet (control group) as well containing Bt protein (experimental group).
Results: Molecular analyses such as immune Dot blot, SDS-PAGE, ELISA and PCR, confirmed the absence of Cry1Ac protein in blood and urine samples of rats, which were fed with Bt protein in their diet. Furthermore, histological studies showed that there was no difference in cellular architecture in liver, heart, kidney and intestine of Bt and non-Bt diet fed rats. To see the effect of Bt on earthworm two different groups were studied, one with transgenic plant field soil supplemented with grinded leaves of cotton and second group with non-Bt field soil.
Conclusions: No lethal effects of transgenic Bt protein on the survival of earthworm and rats were observed. Bradford assay, Dipstick assay ELISA demonstrated the absence of Cry1Ac protein in the mid-gut epithelial tissue of earthworm. The results of present study will be helpful in successful deployment and commercial release of genetically modified crop in Pakistan.
Keywords: Bt protein, Rat, Earthworm, Cry1Ac, Cotton
Introduction
Genetically modified crops are produced by the addition of genes to genome of plant. It is performed by the bombardment of gold particles or transfer of gene through soil bacterium, Agrobacterium tumefaciens, which contain plasmid vector, or having additional genes [1]. A variety of transgenic plants modified for different traits such as drought, temperature, salinity, insect and herbicide resistance have been developed. Flavr savr tomato was the first recombinant crop to be introduced for sale in USA in 1994 which was modified for longer shelf life. Similarly, wheat and rye hybrid developed in 1876 was first conventional transgenic cereal created by scientific breeders [2]. Wheat was also first transgenic cereal which was developed by three parental species [3].
Genetically modified organisms (GMO) such as Flavr savr tomato, were supposed to be grown in containment area. However after successful trials of Flavr savr tomato GMO Soya and Corn were evaluated in USA fields on large scale (Australian Science Media Center, 2008). The planting of transgenic crops in the world has increased dramatically since the commercialization of Bacillus thuringiensis (Bt) corn in the mid-1990s [4,5]. Bioengineered crops can confer significant advantages to natural enemies over conventional broad-spectrum insecticides [6] and provide effective levels of control of target pests. The impact on environment through introduction of GMO by usual agriculture practice gained interest when first GM crop was introduced in market [7,8]. The concern about GM on biodiversity especially on non-target insects and pests was much highlighted.
In the last few years, some studies examined the biosafety of transgenic crops, especially the effect of these plants on the fitness of non-target organisms [9]. Most of the available risk assessment information relates to insect-resistant transgenic plants containing genes coding for Bt toxins (corn, cotton, and potato). However, the points discussed here also apply to other insect resistant GM plants. The results of small laboratories conducted on artificial insect diet were found to have minor ecological relevance that’s why precautionary measures should be taken because laboratory condition do not always results the same mechanism by which plant interact with natural enemies in its food web. Furthermore, field surveys with a focus on diversity and abundance of species are needed to understand the effect of insect resistant GM crops on arthropod communities. Mahmood et al., determined that the transgenic crop had no significant effect on non-target insects (insects belonging to orders other than Lepidoptera and Diptera) in field or under storage conditions [10].
Earthworms (Lumbricus terrestris) are considered to be the most important group of soil organisms due to their efficiency in decomposing plant litter and their influence on soil structure [11]. L. terrestris feeds mainly on leaves with roots as a minor component of the diet [12]. As L. terrestris also ingests some soil, it will probably be exposed to Bt toxins from root exudates and plant biomass bound to clay minerals [13]. Rats were subjected to dose of Bt oral, pulmonary, or intravenous. The toxicological database on B. thuringiensis showed no effects on mammalian health attributable to δ-endotoxins. No significant adverse health effects attributable to the test microbe have been reported in these studies in either body weight gain or mortality by clinical observations, or through examination of the test animal’s internal organs at necropsy [14].
The present study was intended to see the effects of transgenic crops on soil microorganisms, animals and other expected hazards due to the introduction of GM crops into the environment. The earthworms and rats were selected for this purpose because of their suitability for this kind of study. Weight gained/loss, mortality/survival, insecticidal gene integration and expression were the main parameters undertaken to investigate effect of transgenic Bt diet in these organisms.
Methods
Experimental design and sampling procedure
Present study was carried out at National Center of Excellence in Molecular Biology (CEMB), University of the Punjab, to check the effects of transgenic crop on soil decaying organisms and rats. In case of earthworm the experiment was comprised on three groups. In first group A, 400 grams (g) of soil was collected from Bt cotton field containing Cry1Ac gene. 176 g Bt cotton leaves which contained a total of 554ug of Bt protein, were crushed with the help of liquid nitrogen and mixed well in soil. Twenty earthworms were added into soil bucket. In second group B, soil bucket contained 400 g soil from Bt cotton without Bt crushed leaves and twenty earthworms. However group C act as a control group having non-Bt soil taken from CEMB nursery with twenty earthworms. Water was sprinkled in all soil buckets to wet the mud, which was necessary for earthworms on regular basis.
Weight
Ten earthworms were weighed from each group and their average increase or decrease in weight was calculated.
Soil Aeration
After every ten days the soil was shuffled and re-shuffled with hands in order to aerate the soil properly and to check the mortality of earthworms.
Dissection of Earthworms
One earthworm from each group was dissected at interval of ten days. Their gut tissue and skin tissue was cut for protein and DNA extraction.
Extraction of DNA from Earthworm
DNA from earthworm was extracted by using the method devised by Chomczynsk, 1997. 100 mg of earthworm’s skin tissue was homogenized with the help of liquid nitrogen and stored in their respective labeled eppendorf. 1 mL DNAzol reagent was added in each eppendorf and mixed well by vortexing. After 15 minutes it was centrifuged at 4˚C, 13362 rcf/10 min. 500 µL of 100% ethanol was added to supernatant, mixed by inverting 5-8 times and placed at room temperature for 1-3 minutes until the solution became cloudy. After incubation was completed the mixture was centrifuged at 4˚C for 2000/5 min. Supernatant was discarded and DNA in the pellet was washed twice with 800 µL of 75% ethanol. At each wash, DNA pellet was suspended in ethanol by inverting the tubes 3-6 times. The 1.5 mL tubes were stored vertically for 1 minute to allow the DNA to settle at the bottom and centrifuged again for 371 rcf/5 min. at 4˚C. The DNA pellet was air dried for 15-20 minutes and then resuspended in 8mM NaOH. DNA concentration was checked on 0.8% gel.
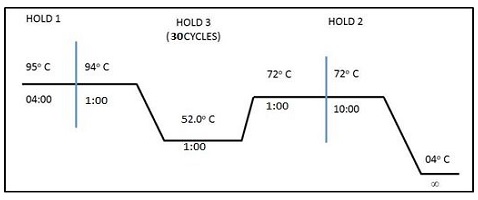
Polymerase Chain Reaction (PCR)
PCR was carried out using specific primers of Cry1Ac gene. DNA extracted from earthworm was used as template in PCR analysis. PCR was carried out in reaction volume of 20 µL containing the genomic DNA template 100 ng, forward and reverse primers 50 pM each, dNTPs 200 µM, 1X PCR Buffer (50 mM KCl, 1.5 mM MgCl2 and 10 mM Tris-HCl) and Taq Polymerase 1 unit. PCR tubes containing above reaction mixture were incubated on a thermo cycler (M.J. Research). The PCR program followed as:
Protein Extraction
About 100 mg tissue from earthworm of six dissections from A, B and C groups were taken, labeled properly and ground to fine powder in liquid nitrogen. The powder was transferred in Eppendorf tubes and 300 µL of ice-cold extraction buffer (10 mM Tris HCl pH 7.5, 40mM EDTA, 150mM NaCl, 10% Glycerol, 1 mg/mL DTT and 1mM PMSF) was added. The tube was kept on ice for 20 minutes and centrifuged at 4°C for 5 min at 18188 rcf. Supernatant was transferred in new eppendorf tube containing extracted protein. The concentration of extracted protein was estimated by the method described by Bradford [15]. 10 µL extracted protein was added to 790 µL 1X PBS and 200 µL BioRad dye. Optical density (O.D) was measured at 595 nm using spectrophotometer.
Quantification of Cry1Ac protein
Cry1Ac protein was quantified by Dot blot procedure. 1 µL of extracted protein from each sample was spotted on nitrocellulose membrane (Hybond-C) with positive Cry1Ac and negative control. The membrane was blocked with blocking solution (5% skimmed milk in PBST) for one hour at 37°C or overnight at 4°C. This was followed by incubation in primary antibody diluted at 1:1000, for one hour at 37°C. After three washings with 1X PBS (15 minutes each) the membrane was incubated in secondary antibody diluted at 1:10,000 for 1 hour at 37°C. Membrane was washed with 1X PBS three times (15 min each) and color was developed using DAB (Diaminobenzidine) as substrate. The membrane was finally washed with distilled water and dried on filter paper.
Selection of Rats
Two groups of rats (Sprague dowley sp.) were selected for 136-day feeding trials. Experimental group contained six rats (3 males and 3 females) which were fed on transgenic diet. Control group also comprised of six rats (3 males and 3 females), which were fed on non-transgenic diet. Three males and three females of experimental group were placed separately in two different cages. Same with control group so that there were four cages and each cage had three rats.
Composition of Diet
For animal feeding tests diet needs to be formulated in which most of the dietary protein was derived from the Bt transgenic crop. All the ingredients were mixed well and cut into equal sized blocks/cakes nearly 130 g and baked in oven at 45˚C. One diet cake was supplied to both groups of rats on regular basis.
Experimental and Control Rats
Rats having closely similar weights were chosen.


Blood Sampling
Blood sample was collected from each rat at intervals of 15 days and checked for the presence of Cry1Ac protein. 50 µL blood was taken carefully from the tail vein with the help of syringe and saved in respective 1.5 mL containing 20 µL 0.5 mM EDTA to avoid blood coagulation. Sample was centrifuged at 15682rcf/15min at 4˚C and serum was pipetted out for dot blot ELISA and SDS-PAGE analysis.
Urine Sampling
In feeding experiment, urine sample was collected for the determination of Bt protein. Sample was collected with the help of metabolic cages. Placing rats in these metabolic cages separately for 24 hours and urine was collected from the lower fixed tube.
Dissection of Rats
One male and one female rat from transgenic and control group respectively were dissected after 138 days of feeding trials. The organs were profuse with PBS through cardiac puncture with the help of syringe and the blood was collected for biochemical analysis.
Morphology of Organs
To access the performance of genetically modified Bt rice under feeding trials, morphological variations in terms of organ structure and maturity were observed.
Biochemical Tests
Blood serum was sent to Zenat Laboratory, Lawrence road, Lahore for six different biochemical analyses i.e., Blood urine nitrogen, Alanine transferase, Aspartate transferase, Creatinine, Lactate dehydrogenase and Cholesterol in order to check the efficacy of vital organs function.
Histological Studies of Bone Marrow Cells of Rats
For the studies of Bone marrow cells, the rats samples were sent to Zenat Laboratory, Lawrence road, Lahore for histological studies of Bone marrow cells i.e. WBC, Neutrophiles, Lymphocytes and Platelets to check the Immunological system of vital organs function.
Dissection of Rats
One male and one female rat of transgenic and control group were dissected respectively after 138 days of feeding trials. Organs were washed using Phosphate Buffer Saline (PBS); heart was perfused by cardiac puncture. Three animals from each cage were sacrificed. Following vital organs were cut with sterilized blade for their morphological and histological studies: Heart, kidney, liver, spleen and intestine.
Morphology of Organs
In order to access the performance of genetically modified Bt cotton under feeding trials, morphological variations in terms of organ structure and maturity were observed.
Histological Analysis of Organs
Heart, liver, spleen and intestine sections were cut and stained for histology. Structural and functional changes were observed through fluorescence microscopy. Specific organs were obtained and cut into small pieces carefully and placed in 4% paraformaldehyde solution for preservation overnight at room temperature with constant shaking in their respective 15 mL tubes.
Processing of Tissues
Tissue sections of spleen, liver, heart and intestine were passed through alcohol grades for dehydration. Treatments of alcohol were done at room temperature with shaking after 5-10 minutes. After dehydration in ethanol grades the organs were wrapped with aluminum foil in culture tubes and heart, intestine and liver sections were treated by xylene twice for 30 minutes.
Paraffin Block Preparation
Two 50 mL falcon tubes for one section were filled with paraffin wax, and placed in shaking water bath set as 72˚C. When paraffin was completely melted tissues were removed from xylene and immersed in molten paraffin in culture tube and incubated at 72˚C for 30 minutes. After 30 minutes, each tissue was immersed in other falcon tube containing molten paraffin and again incubated for another 30 minutes at 72˚C. After paraffin infiltration, paraffin blocks of tissues were prepared. Molten paraffin was poured into mould cleaned with xylene. Tissue was placed in the center of mould with cut side towards bottom. Cassettes were labeled with name of section and date. Appropriately labeled cassette was put over tissue in correct orientation. Molten paraffin was poured over cassette to fix it and paraffin was allowed to solidify at room temperature. Then placed at 4˚C for half an hour, tissue blocks were removed from mould, wrapped in aluminium foil and stored at 4˚C.
Preparation of Tissue Sections
Microtome was set at 47˚C. Tissue blocks were put in refrigerator at 4˚C before sectioning for 30 minutes. After this, microtomy of each tissue was done and sections were taken on glass slides, labeled and stored at room temperature.
Hematoxylene and Eosin Staining of Slides
Tissue sections on the slides were treated with xylene for three minutes twice. After xylene treatment the tissues sections were passed through alcohol of different grades i.e., 100%, 95%, 85% and 70% carefully for 3 minutes. Then the slides having tissue sections were washed with distilled water and treated with hematoxylene for fifteen minutes. Sections were treated with Eosin for 1 minute. The slides were washed containing sections with running water and again treated with ethanol. Finally cytosine mount was added to the section and observed under the microscope.
Results
Weight of Earthworm
The weight of Earthworms was recorded at the interval of ten days for the total period of fifty days on regular basis. All the earthworms showed increase in weight with the passage of time whether they belong to Bt+ soil or Bt – soil (Figure 1). However, no lethal effect on the health of these experimental earthworms was observed.
Polymerase Chain Reaction (PCR)
This genomic DNA was amplified by using Cry1Ac specific primers and resolved on 1% agarose gel. Not a single sample of earthworm tissue showed amplification Cry1Ac (565 bp) expect positive control (Figure 2).
Protein Estimation
From the epithelial tissue of earthworm protein was extracted and concentration was estimated as the method described by Bradford (1976). The estimated protein was quantified by using Cry1AC ELISA kit. Results showed that none of the sample have Cry1AC protein in the sample of earthworm (Table 1).
Immunoblot Assay
Expression of Cry1Ac protein from the earthworm fed on transgenic Bt diet and non Bt diet was attempted to quantify by Immunoblot assay. Total protein was extracted from earthworm fed by transgenic Bt diet as well as non-transgenic Bt diet. This extracted protein was spotted on nitrocellulose membrane (Hybond-C) along with quantified positive control. Purified protein from Bacillus thuringiensis. HD-73 was taken as positive control. Only protein in positive control was detected (Figure 3). No appearance of color was observed in any sample which showed complete absence of Cry1Ac protein in experimental and control. The results were compared with a positive control (Figure 3).
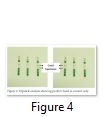
Dipstick Assay for Bt protein in Earthworm
Dipstick assay was performed to check the presence or absence of Cry1Ac on initial level. Only positive band was appeared in control as shown in Figure 4, which indicated the absence of Bt protein in the samples of Earthworm gut.
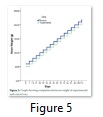
Effect on Weight of Rats
Weight of all twelve rats was monitored with the help of weighing balance after every seven days. The bar diagram of the combined weight of rats of experimental male and female and control male and female groups indicated that the growth of rats was probably equal during the whole study. There was no significant difference in weight of control and transgenic rats and all the rats experienced good growth with the passage of time (Figure 5).
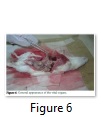
Morphological Studies of Rats
After dissection of four rats, morphology of vital organs was studied for one male and one female from experimental group while one male and one female from control group. However, there was no significant difference e.g., color, texture, organ serosis, appearance of spots etc. in the general appearance of these organs (Figure 6).
Physiological Studies
After H & E staining of the organs, it was clearly seen that there was no difference in the physiological organs of experimental and control animal groups.
Biochemical Tests
Biochemical tests were performed for measuring the amount or activity of a particular enzyme or protein in the sample of blood, urine and other tissue from the body. Six different types of biochemical tests (Blood urine nitrogen, Alanine transferase, Asparate transferase, Creatinine, Lactase dehydrogenase and Cholesterol) were done from the rat’s serum. Results of these tests have clearly shown that there was no difference between experimental and control animals (Table 2).
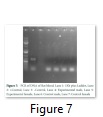
Polymerase Chain Reaction
The genomic DNA was used as template using Cry1Ac specific primers and amplification was confirmed by running the PCR product on 0.8% agarose gel. Not a single sample of rat’s blood showed amplification at 565 bps band of Cry1Ac (Figure 7).
Dot blots of Rat’s Blood and Urine Samples
Expression of Cry1Ac protein in blood and urine samples of experimental and control rats were quantified by dot blot method. Blood serum/urine was spotted on nitrocellulose membrane (Hybond-C) for dot blot analysis along with quantified positive control. There was no detection of Cry protein in blood and urine samples of rat. Moreover, total protein in the blood serum and urine was calculated by using Bradford assay (Table 3 & 4).
SDS-PAGE
SDS-PAGE is an excellence method to check our required protein. Total protein was extracted from rat’s blood samples along with control and about 20 µg of extracted protein was loaded on 12% polyacrylamide gel. Cry1Ac protein was not observed in all the blood samples (Figure 8).
Histology of Organs
After staining slides were observed and no significance difference was noticed. Slides of different organ samples were observed and no considerable differences were noticed.
Heart Tissues
Organs of both, transgenic rat and control rat were subjected to microtome section and after staining observed under microscope and it was finalized that there was no difference in tissues of Bt and non Bt fed organisms (Figure 9).
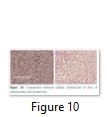
Liver Tissue
Liver of both, transgenic rat and control rat were subjected to microtome section and after staining observed under microscope and it was finalized that there was no difference in tissues of rats that eat Bt and non Bt diet (Figure 10).
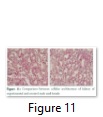
Kidney Tissue
Kidney of rats, after dissection was subjected to microtome section and it was observed that both kind of diet showed no effect on kidney of rats (Figure 11).
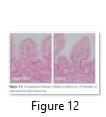
Intestine Tissue
Intestine microtome also resulted that there is no considerable difference between control and transgenic rats (Figure 12).
Data and Tables
Discussion
It is very important to check the effect of transgenic crops on earthworm because they improve the physical structure, fertility, water filtration rates and absorption rates of soil and also helping the soil to drain better. Less runoff equals less watering and less erosion. The tunneling activity improves soil aeration, porosity, and permeability. Earthworms also increase moisture absorption and moisture available to plants. Castings have the ability to absorb moisture from the air and hold it in a manner that plants can use. In order to evaluate the effect of transgenic diet on earthworms, weight was measured on regular basis. All earthworm gained weight during the whole length of experiment. Statistical significance between experimental and control groups was computed using Student t-test. Results indicated that transgenic diet did not significantly change the weight of experimental and control earthworms. These results were in agreement with the results previously reported [16,17]. No any lethal effects on earthworm were found during the experiment, as reported earlier [13,18,19].
Similarly, protein isolated from mid gut epithelial tissues of earthworm groups A, B and C at different intervals clearly indicated the absence of this specific protein except appearance of positive control. The brush border epithelial cells of mid gut do not have receptors for Cry1Ac protein so it is believed that either proteins were degraded or have passed through the whole digestive tract without causing any toxicity. PCR of all the samples were negative except positive control for Cry1Ac (Figure 2). This result indicated that Cry1Ac gene is not integrated and amplified in the genome of earthworm. These finding are in agreement with [20] who reported no toxic effects of Cry proteins on woodlice, collembolans, mites, earthworms, nematodes, protozoa. Protein samples were taken from earthworm epithelial tissues, it did not show required band, therefore negating the presence of Cry1AC protein. Similar findings were reported by [21] to determine the effects of the Bacillus thuringiensis Cry1Ab toxin on the earthworm. Bt protein caused no effect on earthworms due to three main reasons. Bt protein required 9.5 pH to act but pH of intestinal environment is 7.5-8.0 [7]. Specific protein receptor are necessary for binding of Bt protein, which are absent in earthworm.
Weight measurement was the first parameter to check the effect of Bt protein on rats. All rats gained weight during the whole length of experiment (Figure 5). All data was reported as arithmetic means ± SE and analysis of variance at 5% level of significance. Statistical significance between groups was computed using Student t-test. Results indicated that Bt protein did not significantly change weight of experimental and control rats throughout the experiment. Moreover, Final reading indicated that all the rats showed nearly equal increase in weight from their initial weight. These results were in agreement with the results of [16], who reported that GM potato based diets did not cause any harmful effects on growth rates and weights of rats.
In this study, six biochemical tests i.e., Blood urea nitrogen, Alanine transferase, Aspartate transferase, Creatinine, Lactate dehydrogenase and Cholesterol were performed. From these biochemical tests it was clear that rat’s enzyme function normally at the same level just like human enzymes and the functions of organs of experimental group (male, female) showed no difference compared to control (Table 2).
Blood sampling after every fifteen days interval was done to check the effect of Bt protein on rat’s blood using molecular techniques i.e., PCR, Dot blot analysis and SDS-PAGE. Results from dot blot for detection of Cry1Ac protein indicated that Cry1Ac protein was present in rats’ blood. However serum contained heavy amount of total soluble protein (Table III), but Cry1Ac protein was not detected in any sample. Positive and negative controls were also spotted on nitrocellulose membrane along with test samples there was detection of Cry1Ac protein in positive control. This may be due to the effect that Cry1Ac protein was not transported in blood as result of digestion. In this study, Urine sampling at the end of experiment was used for Dot blot analysis. Results were negative for the presence of Cry1Ac protein although urine has minute quantity of total soluble protein (Table IV), but Cry1Ac protein was not detected in the sample (Figure 6). Similar results were found by Hammond [22] reported that in feeding experiments of Bt corn on rats, urine sample was collected for the determination of net protein utilization and nitrogen balance showing no harmful effects. SDS-PAGE of extracted protein separated at 68 kDa band of protein, which is a truncated Cry1Ac toxin from integrated Bt gene. This absence of 68 kDa band indicates absence of gene in experimental and control group compared (Figure 8). PCR of all the samples were negative except positive control for Cry1Ac. This result indicated that Cry1Ac gene is not stably integrated and amplified in the genome of rat and earthworm. As blood samples were taken from both groups of rat and epithelial tissues were taken from all three samples for DNA isolation and PCR, negating the presence of Cry1Ac gene (Figure 7). Thus according to the results there is no evidence that Cry1Ac protein in diet cause any change in the genome of organisms. A 28 days toxicity test of Cry32Ab1 and Cry35Ab1 was conducted by [23], Bt protein was administered orally in a ratio of 54% of the pure Cry34Ab1 protein in the diet, in a separate study the diet given was in a ratio of 54% Cry35Ab1 and 36% of Cry34Ab1 to mice. Pathology, hematology clinical chemistry of the blood was taken into account showing no significant difference in the body weight and other parameters showing that Cry34Ab1 and Cry35Ab1 had no toxic effect on mice. Histological analysis were done on spleen, liver, heart and intestinal sections. The results obtained clearly shown that no significant difference was observed in the cellular architecture of the transgenic and control groups of rats (Figures 10, 11, 12 & 13). Transgenic cotton risk assessment was done using different molecular and biochemical techniques, the results clearly showed that there were no significant differences in the control diet fed animals and transgenic diet fed animals [24]. From the results presented in this paper it can be concluded that Cry1Ac transgenic cotton have no hazardous effect on animals. The information provided by this study will allow other researchers to utilize Cry1Ac gene in different crops against lepidopteron insects. It can prove to be a milestone in economy of our country.
Acknowledgements
References
- Foster K, Foster H, Dickson J. Gene therapy progress and prospects: Duchenne muscular dystrophy. Gene therapy, (2006); 13(24): 1677-1685.
- Finkel E. Australia’s new era for GM crops. Science, (2008); 321(5896): 1629.
- Bates SL, Zhao J-Z, Roush RT, Shelton AM. Insect resistance management in GM crops: past, present and future. Nature biotechnology, (2005); 23(1): 57-62.
- Cannon RJ. Bt transgenic crops: risks and benefits. Integrated Pest Management Reviews, (2000); 5(3): 151-173.
- Shelton AM, Zhao J-Z, Roush RT. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annual review of entomology, (2002); 47(1): 845-881.
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annual review of entomology, (1998); 43(1): 701-726.
- James C (2001) Global Review of Commercialized Transgenic Crops: 2001 (ISAAA Briefs No. 24 preview). International Service for the Acquisition of Agri-biotech Applications, Ithaca, NY.
- Dale PJ, Clarke B, Fontes EM. Potential for the environmental impact of transgenic crops. Nature biotechnology, (2002); 20(6): 567-574.
- Groot AT, Dicke M. Insect‐resistant transgenic plants in a multi‐trophic context. The Plant Journal, (2002); 31(4): 387-406.
- Rahman M-u, Rashid H, Shahid AA, Bashir K, Husnain T, et al. Insect resistance and risk assessment studies of advanced generations of basmati rice expressing two genes of Bacillus thuringiensis. Electronic Journal of Biotechnology, (2007); 10(2): 241-251.
- Dunger W, Fiedler H (1997) Methoden der Bodenbiologie–Gustav Fischer Verlag. Jena.
- Lee KE Earthworms: their ecology and relationships with soils and land use. (1985); 144 Academic Press Inc. USA
- Saxena D, Flores S, Stotzky G. Vertical movement in soil of insecticidal Cry1Ab protein from Bacillus thuringiensis. Soil Biology and Biochemistry, (2002); 34(1): 111-120.
- McClintock JT, Schaffer CR, Sjoblad RD. A comparative review of the mammalian toxicity of Bacillus thuringiensis‐based pesticides. Pesticide Science, (1995); 45(2): 95-105.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, (1976); 72(1-2): 248-254.
- El Sanhoty R, Abd El‐Rahman AA, Bögl KW. Quality and safety evaluation of genetically modified potatoes Spunta with Cry V gene: Compositional analysis, determination of some toxins, antinutrients compounds and feeding study in rats. Food/Nahrung, (2004); 48(1): 13-18.
- Poulsen M, Kroghsbo S, Schrøder M, Wilcks A, Jacobsen H, et al. A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food and Chemical Toxicology, (2007); 45(3): 350-363.
- Ahl Goy P, Warren G, White J, Privalle L, Fearing P, et al. Interaction of an insect tolerant maize with organisms in the ecosystem. Mitteilungen-Biologischen Bundesanstalt Fur Land Und Forstwirtschaft, (1995); 50-53.
- Zwahlen C, Hilbeck A, Howald R, Nentwig W. Effects of transgenic Bt corn litter on the earthworm Lumbricus terrestris. Molecular Ecology, (2003); 12(4): 1077-1086.
- Icoz I, Stotzky G. Fate and effects of insect-resistant Bt crops in soil ecosystems. Soil Biology and Biochemistry, (2008); 40(3): 559-586.
- Van Der Merwe F, Bezuidenhout C, Van Den Berg J, Maboeta M. Effects of Cry1Ab transgenic maize on lifecycle and biomarker responses of the earthworm, Eisenia Andrei. Sensors, (2012); 12(12): 17155-17167.
- Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food and Chemical Toxicology, (2004); 42(6): 1003-1014.
- Juberg DR, Herman RA, Thomas J, Brooks KJ, Delaney B. Acute and repeated dose (28 day) mouse oral toxicology studies with Cry34Ab1 and Cry35Ab1 Bt proteins used in coleopteran resistant DAS-59122-7 corn. Regulatory Toxicology and Pharmacology, (2009); 54(2): 154-163.
- Malatesta M, Boraldi F, Annovi G, Baldelli B, Battistelli S, et al. A long-term study on female mice fed on a genetically modified soybean: effects on liver ageing. Histochemistry and Cell Biology, (2008); 130(5): 967-977.