Full Length Research Article
Antimicrobial activities, pollen diversity and physicochemical properties of natural honey from Southeastern Anatolia of Turkey
Menderes Cenet*,1, Adnan Bozdogan2, Gokhan Sezer1, Leyla Acar1, Zeynep Ulukanli1
Adv. life sci., vol. 4, no. 2, pp. 47-54, February 2017
*- Corresponding Author: Menderes Cenet (Email: mcenet@osmaniye.edu.tr)
Authors' Affiliations
2- Department of Food Engineering, Osmaniye Korkut Ata University, Karacaoglan Campus, Osmaniye – Turkey
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Honey, a natural sweetener, is produced from the nectar of many plants. The pollen diversity, physicochemical properties, and antimicrobial activities were analyzed in honey samples from Mardin (Southeastern Anatolia).
Methods: The melissopalynological method was used to identify and enumerate the pollen granules. Analytical methods and agar well diffusion assays were employed for the determination of some quality parameters and the antimicrobial potential of honey samples, respectively.
Results: The pollen composition consisted of 27 taxa belonging to 13 families. The origins of all honey were determined as the multifloral sources. The most predominant taxa were mainly Hedysarum sp., Carduus sp., Melissa officinalis, Gossypium hirsitum, Paliurus spina-christi, Salix sp. and Pimpinella anisum. The secondary pollen taxa were Hedysarum sp., Trifolium sp., Astragalus sp., Salix sp., Paliurus spina-christi, Asphodeline sp., Centaurea sp., Carduus sp., Zea mays and Cistus sp., respectively. Asphodeline sp. as a secondary pollen taxon in a honey sample could be considered as the first report. The pH, total acidity, brix, refractive index, electrical conductivity, moisture and L, a, b values of the samples varied from 3.75 to 4.28, 30 to 42, 67.3 to 85.70, 1.45 to 1.50, 12.40 to 31.61, 0.24 to 0.90, 47.81 to 57.59, -0.94 to 4.31, 20.37 to 31.28, respectively. Antimicrobial activities of the honey specimens were also effective on five bacterial species and two yeast species.
Conclusions: Honey samples from Southeastern Anatolia revealed a good diversity of pollen granules. The rich multiflora of honey increases not only its nutritional quality as well as antimicrobial potential on various clinically important microorganisms.
Key words: Total Soluble Solids, Melissopalynology, Pollen analysis, Honey, Antimicrobial activity, Mardin
Introduction
Honey, is a natural sweetener, is produced from the nectar of many plants. The use of this sweetener has been back to earliest times owing to their taste and etc. Various important compounds such as amino acids being the primary elements of proteins, vitamins and macro and micro minerals for human health are also present in this natural beehive product. Variation of the ingredients of honey has been based on some biotic parameters e.g. plant species and abiotic parameters e.g. climatic, habitat and etc. [1]. Turkish flora is very rich in plant diversity and includes about 12000 vascular plants. The ecological differences in seven regions of Turkey provide very good diversity in nectarous plants for honey production. The quality parameters, origins and biological activities of honey specimens have been reported from some regions of Turkey [2,3]. In Turkey, honey production is about 94.245 tons and exported to various countries in the world. Tracking the floral source of honey is the fundamental and traditional parameter affecting the quality, sensory and various health benefits of honey. According to literature, there has been no previous report on the pollen spectrum, the physicochemical quality parameters and the antimicrobial potential of honey samples from Mardin (Southeastern Anatolia Region of Turkey). Hence, the objective of this study is to determine the mellissopalynological, physicochemical and antimicrobial properties of honey samples.
Methods
Sample collection
Mardin province (Southeast Anatolia) consists of seven districts (36º 55´ – 38º 51´ North latitudes to 39º 56´ – 42º 54´ East longitudes). The east of Mardin province has the terrestrial and the west with a Mediterranean climate. The season is very hot and arid during summer, and it is rainy and cold during winter season. It has been estimated that a total of 621 plant species have been recorded from the natural habitats of Mardin districts. Sixty-one are reported as the endemics in identified species. Five districts (Midyat, Nusaybin, Merkez, Dargeçit, Ömerli) of the Mardin are the most known districts for honeybee keepings with regard to the nectarous plant diversity. In addition, Mardin is a good agricultural area with various crops such as Triticum sp., Gossypium hirsitum, Corylus sp., Olea europeae, Hordeum vulgare, Zea mays, Oryza sativa, Cicer arietinum, Lens culinaris, Vitis sp., Pistacia vera, and Helianthus annuus. A total of seven honey samples were collected from beekeepers in the districts of Mardin; Midyat-Bahçe, Midyat-Merkez, Mazıdağı, Nusaybin, Ömerli, Dargeçit, Savur. All samples were retained in sterilized jars and then stored in fresh and dry place until analysis.
Pollen analyses, identification and counting of pollens
The preparation of the pollen slides from honey samples was employed using the melissopalynological method [4,5]. In brief, honey (10 g) was diluted with distilled water (20 ml). Test tube was initially sealed with the parafilm and then retained at 45 °C for 15 min. Dissolved samples were centrifuged at 6500 rpm for 20 min, followed by discarding the upper phase. The resultant sediment was mixed with vortex and then, pollen slides were prepared using glycerin/gelatin mixture (1/1.5, v/v) [6]. Treated slides were briefly kept on hot plate, and then left at inverted position to fix the samples. Pollen grains were counted using a light microscope (Olympus CX21). Identifications of the grains were based on the reference books and various literature. The genus and/or species of pollen grains was also identified using the reference slides from the palynology laboratory and those prepared from plant species collected from the Mardin region. Image of each taxon was photographed with a Euromex PB 4161. The percent of pollens on each slide was calculated and evaluated on the basis of following scala suggested by Louveaux et al. [5]. ≥45 % and more: dominant; 16-44 %: seconder; 3-15: minor and <3 %: trace.
Physicochemical analyses
The pH of honey solutions (10 g/75 ml, w/v) was measured using a pH meter (Thermo Scientific, Singapore). Total acidity were determined in honey solutions (10 g/75 ml, w/v) using a titrimetric method. Total soluble solids of honey samples (°Brix) were measured at 20 °C using a digital refractometer (Krüss Optronic, Germany). Electrical conductivity was measured in a (10 g/75 ml, w/v), using Hanna EC 215 model conductivity meter (Hanna Instruments, USA) [7]. Refractive index of honey samples were measured using a digital refractometer (Krüss Optronic, Germany) (at 20 °C) and corresponding moisture content (%) was calculated using the relationship between refractive index and water content. For colour measurement, the samples were heated at 50 ºC for 60 min to dissolve the crystal structure. Samples were placed in a plastic container and covered with a plastic plate. The measured layer was 1 cm thick. Colour (L, a and b parameters) was determined with a Conica Minolta colorimeter (Chroma Meter CR-400 Japan) [8].
Antimicrobial screening
Staphylococcus aureus ATCC 29213, Staphylococcus aureus ATCC BAA-977, Enterococcus casseliflavus ATCC 700327, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumoniae ATCC 700603, Enterobacter hormaechei ATCC 700323, Candida parapsilosis ATCC 22019 and Candida albicans ATCC 14053 were included as the test microorganisms. Antimicrobial activities of the honey samples were carried out using the agar well-diffusion assay [9]. Mueller Hinton Agar medium (MHA, CM 337, Oxoid Ltd. Basingstoke, UK) for bacterial species and Potato Dextrose Agar for yeast species (20 ml) was poured into petri plates. After solidifying, a total of 0.1 ml of overnight culture of the microbial culture, which was adjusted to the Mac Farland Unit (0.5), was aseptically transferred on to the medium and spread with drigalski spatula. On each inoculated medium, four wells were punched with a sterile cork borer with a diameter of 8 mm. Each honey sample was dissolved in 0.1 % saline solution (w/v) and prepared at the following concentrations; 15, 35, 55 and 75 % (w/v). An aliquot of 0.05 ml from each concentration was aseptically loaded into agar-well. Agar media itself and the standard antibiotics agents; Penicillin 10 U (P10); Cefuroxime 30 mcg (CXM30); Trimethoprim/Sulfamethoxazole 1.25/23.75 mcg (SXT 25); Teicoplanin 30 mcg (TEC30) Bioanalyses Ltd. UK; Nystatin (NS 100 U, Oxoid, UK) were used as the controls. The diameter of the inhibition zones were measured after 24 h at 35.5 °C. Antimicrobial assays were carried out in triplicate.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA). The comparison of the mean difference was based on the Tukey test (P<0.05). SPSS for Windows 18.0.0 (SPSS Inc., Chicago, USA) was used for the processing the data.
Results
Pollen diversity of honey samples
The composition and photomicrographs of the pollen grains were shown in Table 1 and Figure 1, respectively. In the present study, a total of 27 taxon were determined belonging to the following families; Apiaceae, Asteraceae, Convolvulaceae, Fabaceae, Fagaceae, Juglandaceae, Lamiaceae, Liliaceae, Malvaceae, Poaceae, Rhamnaceae, Rosaceae and Salicaceae. The diversity of pollen taxa varied from 7 to 12 depending on the honey specimen.
Antimicrobial activities of honey samples
The antimicrobial activities of the honey specimens were shown in Table 3.
Tables & Figures
Discussion
The presence of dominant and seconder pollens of nectarous plants are known to be the fundamental determinants for finding the origin of honey and consequently affecting the quality parameters [4].
All honey specimens collected from Mardin region had no predominant pollen; however, the secondary, moderate and minor levels of pollens were present in the samples.
As shown in Table 1, Hedysarum sp. (Midyat-Bahçe, Nusaybin, Ömerli), Trifolium sp., Astragalus sp., Salix sp. (Mazıdağı, Nusaybin, Ömerli), Paliurus spina-christi (Midyat-Bahçe, Ömerli), Asphodeline sp., Centaurea sp., Carduus sp., Zea mays (Mazıdağı, Nusaybin), and Cistus sp. (Midyat-Merkez) were determined as the secondary taxon. Melissa officinalis, Salvia sp., Ceratonia siliqua, Gossypium hirsitum, Convolvulus arvensis, Senecio sp., Pimpinella anisum, Crataegus monogyna, Castanea sativa, Astragalus sp., Salix sp., Carduus sp., Zea mays, Hedysarum sp. and Paliurus spina-christi were the minor taxon (Table 1). The present results indicated that Hedysarum sp., Carduus sp., Melissa officinalis, Gossypium hirsitum, Paliurus spina-christi, Salix sp. and Pimpinella anisum were the most common taxon in all specimens. Trifolium sp., Astragalus sp., Hedysarum sp., Centaure sp., Carduus sp., Paliurus spina-christi and Salix sp. were the secondary type in most of the specimens. The frequent occurrence of many species in Asteraceae and Fabaceae were also well-reported in honey specimens from many regions of Turkey as well as in other countries. The pollen belonging to Asphodeline sp. as a secondary taxon in honey sample could be considered as the first report. All honey specimens Mardin region were classified as the multifloral according to the generic and species level and these findings indicated that honeybees collected the nectar from various plant species. The pollen spectra, which is important for the consumer’ preference, and also influence the quality of honey.
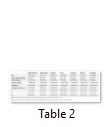
The significant differences were observed in honey samples with regard to the moisture content (p<0.05). The moisture of five honey samples ranged from 12.4 to 15.13 %, whereas two samples Midyat-Merkez (31.28 %) and Mazıdağı (31.61 %) exceeded 20 %, which were higher than the limit of Turkish Standards as well as the European Community Directive [10]. Various parameters such as honey production method, harvesting time, biotic and abiotic factors (botanical origin, climatic and geographical conditions) have significant influences on the moisture content of honey [11,12]. Increases in moisture content of honey favor the growth of microorganisms and limit the shelf life of honey [7,10,12,13]. High percent of moisture content in the present results could be related with those aforementioned factors. In previous studies, more or less similar moisture content of honey samples were reported from different geographical sources (varying from 7.99 to 23.4 %) [13]. It has been known that differences in the acidity of honey samples were related to the season and type of plant species where honeybee collected the nectar [14]. In addition, some organic acids e.g. gluconic, pyruvic, malic and citric acid, lactones or esters and some inorganic ions e.g. phosphate and chloride are the important characteristics of the titratable acidity of honey [7,13,15,16]. Total acidity values of honey specimens were statistically significant. As shown in Table 2, TA values of honey samples ranged from 30 to 42 (meq/kg), which were within the acceptable values of total acidity (50 meq/kg) proposed by EU directive 2001/110/CE [17].
The pH value has a great importance during the extraction and storage of honey. The significant effect of pH on texture, stability and shelf life were also reported in earlier studies [16,17]. As shown in Table 2, all honeys were acidic characters ranging from 3.75 to 4.28. Nevertheless, the significant differences in relation to the pH value were not observed in any of the samples examined. The findings of this study were also similar to previous reports [18-20].
Brix was statistically significant due to locations. As shown in Table 2, Brix varied from 67.3% to 85.7% in the samples except honey sample from Midyat- Merkez (67.3%). The differences in honey specimens could be related to climate, floral source and other factors. The refractive index varied from 1.46 to 1.50 and the corresponding moisture content ranged between 12.40% and 31.61%.
Electrical conductivity is one of the best methods for the determination of honey samples from different flora, which is related to mineral matter (total ash), complex sugar, organic acid and protein concentration [21,22]. As shown in Table 2, two samples from Mazıdağı and Nusaybin had electrical conductivity values of 0.90 mS/cm, which were higher than those of the proposed limit of European Community Directive (0.8 mS/cm). In contrast, honey samples from some districts in the investigate region varied between 0.24 and 0.33 mS/cm. More or less similar results were reported by Yucel and Sultanoglu [14].
As could be seen Table 2, the colour parameters such as L (lightness), a (red) and b (yellow) varied significantly due to locations. The colour is known to be an indicator of honey origin, which is linked to the chemical composition, storage time, mineral matter, chlorophyll, carotenoid, tannin and polyphenolic compounds. L value is the indicator of lightness of honey specimens [13,20,23]. It has been known that a (+) or a (-) values indicates the red and green colour in honey samples, respectively. This means that honeybee collects the nectar from different floral origin. The findings of this study were well accordance with the findings of Tornuk et al. [13].
All honey specimens did not reveal any antibacterial effect on B. cereus, E. casseliflavus, M. luteus, K. pneumoniae and P. aeruginosa (data were excluded from the Table 3). On the other hand, the most notable antimicrobial effects were on S. aureus 29213, S. aureus BAA-977, E. faecalis 29212, E. coli 25922, E. hormaechei 700323, C. parapsilosis 22019 and C. albicans 14053. Dose dependent relationships were observed depending on the tested honey specimens. It could be clearly seen that the specimen from Midyat-Bahçe indicated the highest antimicrobial activities on B. subtilis and C. albicans. Honey sample from Nusaybin had also significant antimicrobial effects on S. aureus BAA, E. faecalis, E. hormachei and C. parapsilosis. Furthermore, honey specimen from Dargeçit was also notably active on S. aureus 29213. Honey sample from Mazıdağı showed the highest antimicrobial effect on E. coli. The findings of this study were also well supported with earlier reports indicating that honey rich in pollens belonging to Salix sp. and Zea mays has significant antibacterial effects on a wide range of microorganisms [24,25].
The presence of the multifloral pollen grains of honey samples from Mardin indicates the importance of the region providing a good diversity of plant species for natural honey production. The results of the physicochemical parameters as well as their antimicrobial potential provide could suggest the consumption of honey from Mardin region as a rich source of natural product.
Acknowledgement
The study was partially supported by OKÜ-BAP.
References
- Küçük M, Kolaylı S, Karaoğlu Ş, Ulusoy E, Baltacı C, et al. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chemistry, (2007); 100(2): 526-534.
- Sorkun K, Inceoglu O. Secondary pollens in honey of the central Anatolia region. Doga Bilim Dergisi, (1984); 8382-384.
- Mercan N, Guvensen A, Celik A, Katircioglu H. Antimicrobial activity and pollen composition of honey samples collected from different provinces in Turkey. Natural Product Research, (2007); 21(3): 187-195.
- Maurizio A, Hodges F. Pollen analysis of honey. Bee World, (1951); 32(1): 1-5.
- Louveaux J, Maurizio A, Vorwohl G. Methods of melissopalynology. Bee world, (1978); 59(4): 139-157.
- Çenet M, Toroğlu S, Keskin D, Bozok F. Pollen analysis and antimicrobial properties of honey samples sold in Western Turkey. Pakistan Journal of Zoology, (2015); 47(1): 269-273.
- Gomes S, Dias LG, Moreira LL, Rodrigues P, Estevinho L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food and Chemical Toxicology, (2010); 48(2): 544-548.
- Ulukanli Z, Oz AT, Cenet M. The authenticity of honey and its effect on strawberry fruits. Journal of Food Processing and Preservation, (2012); 36(4): 364-373.
- Cenet M, Ulukanli Z, Bozdogan A, Sezer G, Memis E. The authentication of the botanical origin, physicochemical properties, antioxidant and antimicrobial activities of East Mediterranean honey. Biointerface Research in Applied Chemistry, (2016); 6(6).
- Kahraman T, Buyukunal SK, Vural A, Altunatmaz SS. Physico-chemical properties in honey from different regions of Turkey. Food Chemistry, (2010); 123(1): 41-44.
- Cano C, Felsner M, Matos J, Bruns R, Whatanabe H, et al. Comparison of methods for determining moisture content of citrus and eucalyptus Brazilian honeys by refractometry. Journal of Food Composition and Analysis, (2001); 14(1): 101-109.
- de Rodrı́guez GO, de Ferrer BS, Ferrer A, Rodrı́guez B. Characterization of honey produced in Venezuela. Food Chemistry, (2004); 84(4): 499-502.
- Tornuk F, Karaman S, Ozturk I, Toker OS, Tastemur B, et al. Quality characterization of artisanal and retail Turkish blossom honeys: Determination of physicochemical, microbiological, bioactive properties and aroma profile. Industrial Crops and Products, (2013); 46124-131.
- Yücel Y, Sultanog P. Characterization of honeys from Hatay Region by their physicochemical properties combined with chemometrics. Food Bioscience, (2013); 116-25.
- Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chemistry, (1998); 63(4): 549-562.
- Silva LR, Videira R, Monteiro AP, Valentão P, Andrade PB. Honey from Luso region (Portugal): Physicochemical characteristics and mineral contents. Microchemical Journal, (2009); 93(1): 73-77.
- Terrab A, Recamales AF, Hernanz D, Heredia FJ. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chemistry, (2004); 88(4): 537-542.
- Corbella E, Cozzolino D. Classification of the floral origin of Uruguayan honeys by chemical and physical characteristics combined with chemometrics. LWT-Food Science and Technology, (2006); 39(5): 534-539.
- Ahmed J, Prabhu S, Raghavan G, Ngadi M. Physico-chemical, rheological, calorimetric and dielectric behavior of selected Indian honey. Journal of Food Engineering, (2007); 79(4): 1207-1213.
- Juszczak L, Socha R, Rożnowski J, Fortuna T, Nalepka K. Physicochemical properties and quality parameters of herbhoneys. Food Chemistry, (2009); 113(2): 538-542.
- Terrab A, Dı́ez MJ, Heredia FJ. Characterisation of Moroccan unifloral honeys by their physicochemical characteristics. Food Chemistry, (2002); 79(3): 373-379.
- Belay A, Solomon W, Bultossa G, Adgaba N, Melaku S. Physicochemical properties of the Harenna forest honey, Bale, Ethiopia. Food chemistry, (2013); 141(4): 3386-3392.
- Beretta G, Granata P, Ferrero M, Orioli M, Facino RM. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Analytica Chimica Acta, (2005); 533(2): 185-191.
- Zhao X, Zhang C, Guigas C, Ma Y, Corrales M, et al. Composition, antimicrobial activity, and antiproliferative capacity of anthocyanin extracts of purple corn (Zea mays L.) from China. European Food Research and Technology, (2009); 228(5): 759-765.
- Pop C, Vodnar D, RANGA F, Socaciu C. Comparative antibacterial activity of different plant extracts in relation to their bioactive molecules, as determined by LC-MS analysis. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca Animal Science and Biotechnologies, (2013); 70(1): 86-94.