Full Length Research Article
Green synthesis and antibacterial activities of silver nanoparticles against Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa and Staphylococcus aureus
Moses Enemaduku Abalaka*,1,2, Oghenerobor Benjamin Akpor2, Omorefosa O. Osemwegie2
Adv. life sci., vol. 4, no. 2, pp. 60-65, February 2017
*- Corresponding Author: Moses Enemaduku Abalaka (Email: modorc2005@yahoo.com)
Authors' Affiliations
2- Department of Biological sciences, Landmark University, Omu-Ara – Nigeria
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Over the last two decades infectious agents have become more dangerous, most especially in developing countries, due to their ability to develop resistance against orthodox medicines. Many in these countries are suffering from the debilitating effects of these pathogens without any remedies in sight. The recent researches in nanoparticles derived from medicinal plants seem to be yielding positive results.
Methods: We carried out synthesis of silver nanoparticles from AgNO3 and using Hyaluronic acid as a stabilizing agent to avoid aggregation in green synthesis from Ziziphus spinachristi and Garcinia kola. Transmission electron microscopy (TEM) was used to determine particle size and shape. Disc diffusion technique was used to study the susceptibility patterns of the particles on the test organisms- Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa and Staphylococcus aureus.
Result: The nanoparticles exhibited very high activity against the pathogens at very low concentration and showed remarkable higher activity than the crude extracts and standard antibiotics (control) with very wide zones of inhibition. The zones of inhibition ranged from 12.4±0.11 – 15.1±0.22 for the nanoparticles as against, 8.7±0.21 – 9.2±0.32 for the crude and 10.7±0.22 – 12.7±0.88 for the standard antibiotics.
Conclusion: Green synthesis of silver nanoparticles may give the long awaited breakthrough against these infectious agents to ameliorate, if not completely, win the war against these pathogens.
Key words: Green synthesis, Silver nanoparticles, Ziziphus spinachristi, Garcinia kola
Introduction
Infectious agents (pathogenic microorganisms) are known throughout the world for their ravaging effects on humans, animals and plants are felt everywhere as the history of microbiology itself is that of infection [1]. Human existence has been seriously challenged by these infectious agents otherwise referred to as pathogens. The survival of man has also been a great battle between him and pathogenic organisms. The discoveries of antibiotics beginning from 1928 through the efforts of Alexander Flemming were a milestone to man in his fight against disease causing organisms [2]. Indeed, humans thought the fight has come to end but the organisms soon devised the means of fighting back through resistance to the same antibiotics being celebrated by man [3]. However, man has been the architect of his own failure to conquer the “enemies” of his health once and for all when he, through indiscriminate use, misuse, abuse etc., created avenues for the organisms to become resistant to the drugs. Some microorganisms are naturally resistant to certain antibiotics, but others can acquire resistance through mutations when they are exposed to antibiotics. Antimicrobial resistance, whether natural or acquired, can spread to other microbial species, especially bacterial species, since bacteria can easily exchange genetic material from one to another, irrespective of species [4].
This incidence now has led to new frontiers of efforts to curb the effects of infectious agents through various research efforts including going back to natural products like plants secondary metabolites. It is noteworthy that these efforts are yielding good results but the fight is still on. So many research activities have shown that plant products are useful against microbial pathogens [5-9]. Accordingly, studies have shown that secondary metabolites such as Cardiac glycosides, Polyphenols, Resins, Saponins, and Tannin are present in species of Ziziphus and may have accounted for the antimicrobial activities recorded with the plants [10].
Garcinia kola (also called bitter kola) is a flowering plant. It belongs to the family Clusiaceae or Guttiferae. This plant grows in Benin, Cameroon, the Democratic Republic of the Congo, Ivory Coast, Gabon, Ghana, Liberia, Nigeria, Senegal and Sierra Leone. It grows well in subtropical or tropical moist lowland forests [11]. The fruit, seeds, nuts and bark of the plant have been used for centuries in trivial medicine to treat ailments from coughs to fever [12]. According to a report from the Center for International Forestry Research, Garcinia kola trade is still important to the tribes and villages in Nigeria. Researchers have indicated that Africans use Garcinia kola for purgative, antiparasitic, and antimicrobial purposes. The seeds are chewed to cure bronchial disease, oral-pharyngeal infection, headaches and even colds, and cough [13].
Human beings have opened up so many frontiers in their fight for survival at the hand of disease-causing agents. So far, efforts against “microbial enemies” of human health are increasing, but with less implementation and negligible output. Man needs quick answers to these problems but the organisms are also developing ways of making this fight tougher for man by means of resistance to commonly used antimicrobial agents. One of the ways researchers are trying to mitigate the attacks from pathogens is by targeting drug efficacy and drug delivery through the use of nanoparticles. Due to their minute sizes, nanoparticles are thought to go far in delivering active components of drugs to target organs of the body thereby affecting the survival of the pathogens.
In this study, the green synthesis of silver nanoparticles derived from the medicinal plants Ziziphus spinachristi and Garcinia kola have been used against four pathogens known to cause various ailments in man and other animals.
Methods
Collection of plants materials
Plants were obtained from the villages around the Landmark University, Omu-aran, Nigeria. They were identified and confirmed by a Botanist in the University of Ilorin, Nigeria and voucher specimens (UNILBSC1675 and UNILBSC1676) were deposited in the herbarium for further use.
Preparation of plant materials
The plants parts used were air-dried in a controlled laboratory environment for about 2 weeks with occasional turning and sorting. When well dried plants were pulverized and pounded in laboratory mortar and powdered materials were stored in dry containers until needed. Extraction of plant materials was done using distilled de-ionized water and ethanol.
Synthesis of Ag nanoparticles
Silver nitrate (AgNO3) of analytical grade was purchased from Sigma–Aldrich Chemical Pvt. Ltd. In a typical reaction procedure, 1ml crude extract of each plant was diluted to 300ml by using distilled de-ionized water which made it 3% and 20 ml of this extract solution was mixed with 20 ml 5×10−3M aqueous silver nitrate solution. The mixture had the pH of near neutral of 7.1 and this was maintained throughout the experiments. The resulting mixture was then heated at 85oC with constant stirring for about 4 hours in oil bath which yielded silver nanoparticles. Hyaluronic acid was added as a stabilizing agent to avoid aggregation in green synthesis of these plants- Ziziphus spinachristi and Garcinia kola. The same reactions were repeated for the two plants using various concentrations of AgNO3.
Visible color change was observed for the reaction mixture containing silver nitrate and each of the plants. The spectrum with bands in this range has been associated with the surface Plasmon resonance of nanoparticles synthesis of silver metal. This confirms the presence of nanoparticles from the plants extracts.
Characterization of the nanoparticles
Morphology and size of silver nanoparticles were investigated using transmission electron microscopy (TEM) images. TEM images were obtained on a Ziess Leo 910 transmission electron microscope according to already established method [14]. In this experiment, 10µl of each sample was placed on the carbon coated copper grid and excess of the sample was removed by a blotting paper. We dried the grid using an infrared lamp which has an accelerating voltage of between 40-120 KV. The resultant images were taken using 0.4 nm resolution Gatan SC1000 camera.
Antibacterial activity of crude plants extracts and AgNO3 on the four pathogens
Bioactivities of the crude extracts and the silver nitrate solution were determined by using the disc diffusion method [15]. The sterile paper discs (6mm) impregnated with the plants leaf extracts were suspended in distilled water which were later left to dry under the temperature of 37oC for 24 hours. The test organisms were prepared as suspension in normal saline from nutrient agar plates, the agar plates were grown for 18 hours. Approximately 5ml of nutrient broth was inoculated with the test organism and incubated for 24 hours. 0.2ml from the overnight culture of the organisms was dispensed into 19ml of sterile nutrient broth and McFarland’s standard was used to standardize the culture to 106cfu/ml after 3-5 hours incubation. Swab was used to inoculate the surface of Muller Hinton agar and the nanoparticles impregnated discs were placed on it equidistant from one another and incubated for 24 hours at the temperature of 37oC.
Following the expiration of incubation period, zones of inhibition produced around the discs were measured and recorded. AgNO3 was evaluated the same way to determine its antibacterial activity.
Antibacterial activity of silver nanoparticles on the four pathogens
The antibacterial activity of silver nanoparticles on the four pathogens, was determined using the method described and used by on disc diffusion [16]. The antimicrobial assay was conducted according prescribed protocol in literature [17,18]. The sterile paper discs (6mm) impregnated with silver nanoparticles derived from the two plants extracts were obtained and the procedure described above was followed. All tests were conducted in triplicate.
Results
In table 1, the results of both aqueous and ethanol extracts of Z. spinachristi and G. cola against the test organisms are revealed.
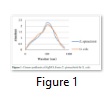
The results showed that the ethanol extracts of both plants had higher activity against the organisms indicating that they may contain more active components than the aqueous extracts. Figure 1 shows the results of the UV–Visible Spectrometric Analysis of green synthesis of nanoparticles form the two plants used in this study. The wavelength on the spectrophotometer indicate the peak of synthesis of nanoparticles from the plants between 450-580nm. Visible color change was observed for the reaction mixture containing silver nitrate and each of the plants. From the table 2 it is evident that the AgNPs obtained from Z. spinachristi and G. kola showed more activity against each of the four pathogens compared to commercially available standard antibiotics.
Tables & Figures
Discussion
The UV–visible spectrometric analysis of green synthesis of nanoparticles from Z. spinachristi and G. kola used in this study revealed the peak of synthesis of nanoparticles from the plants between 450-580nm. Researchers in nanotechnology use various spectroscopic techniques to characterize the nano-composites depending on the extent and dimension of the investigation. Researchers [19-21] for instance, all alluded to the use of UV-visible, X-rayphotoelectron (XPS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to characterize the nanoparticles sizes. In this present study we employed UV visible, and TEM to characterize the sizes of the synthesized nanoparticles. Active components in the form of secondary metabolites contained in these plants materials are believed to be responsible for the reduction of AgNO3 to yield the silver nanoparticles. Our earlier reports on Z. spinachristi have shown the presence of some of these secondary metabolites [10].
Table 1 shows varying actions of the various extracts on the pathogens studied. Ethanol extracts from the two plants were seen to have higher activity against the test organisms, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa and Staphylococcus aureus compared to the aqueous extracts. Interestingly, extracts of Garcinia kola had higher activity against the organisms than the two extracts of Ziziphus spinahristi. Evidently, other researchers had similar results working on the antibacterial activities of Berberis vulgaris and Ziziphus vulgaris [9]. The extent of accumulation of secondary metabolites in a plant may affect the level of antimicrobial activity of such plant. That may have accounted for the differences in the activities of these plants. From table 2 it is evident that the activities of nanoparticles studied in this work outweigh those of crude extracts as well as standard antibiotics. Nanoparticles from G. kola again outclassed those of Z. spinachristi in antibacterial activity. However, nanoparticles from both plants edged the commercial antibiotics, Chloramphenicol and Streptomycin in antibacterial efficacy. Activities of nanoparticles on biological agents are largely due to the minute nature of its particles usually in nanometers (nm) [20]. This minute nature provides very large surface areas for the active components contained therein to come into contact with and penetrate deeper into the microbial cells. Some researchers in their work, reported similar results when they examined the efficacy of plant-mediated synthesized silver nanoparticles against Sitophilus oryzae, an insect pest of Zea mays [22]. Apart from using standard antibiotics and comparing their activities on the bacteria with those of our synthesized silver nanoparticles, we also examined the effect of silver nitrate on the test organisms. As expected, silver nitrate showed activity against the organisms. However, the activity was far below those of crude extracts not to talk of the nanoparticles. This result then means that although silver nitrate has certain antibacterial properties, it is far less as compared with extracts and nanonized extracts. From these experiments (which we conducted in triplicates) we can confidently confirm that silver may not have added to the antibacterial effects of nanoparticles synthesized from these plants. This result is in conformity with the results obtained in an earlier study [21]. The isolates tested in this research, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa and Staphylococcus aureus are well known pathogens responsible for a number of diseases of man, animals, and plants. E. coli causes cholecystitis, bacteremia, cholangitis, urinary tract infection (UTI), and traveler’s diarrhea, and other clinical infections such as neonatal meningitis and pneumonia [23]. The plants Z. spinacrhisti and G. kola could be used to produce useful drugs to combat the diseases caused by these pathogens.
It is concluded that the two plants investigated in this research, Z. spinachristi, and G. kola, have antibacterial activities against Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa and Staphylococcus aureus. The nanoparticles from the plants’ extracts have higher activities against the organisms. Antibacterial effects of the standard antibiotics used as the control are far lower than the silver nanoparticles of the plants’ extracts but higher than those of the crude extracts. Although AgNO3 has antimicrobial properties, its activities on the test organisms are far lower than both the crude extracts and the nanoparticles. Since these organisms are known human pathogens activities of these plants against them may lead to the development of novel drugs capable of treating the infections caused by these organisms.
Acknowledgement
Authors wish to thank the laboratory attendants in the department of microbiology, Federal University of Technology, Minna, Nigeria and Department of Biological Sciences Landmark University, Omu-Aran, Nigeria for their assistance. They also want to acknowledge the Authorities at Federal University of Technology, Minna and Landmark University, Omu-Aran for providing the enabling environment for research.
References
- Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clinical Infectious Diseases, (2004); 39(7): 971-979.
- Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. British Journal of Experimental Pathology, (1929); 10(3).
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. The Lancet infectious diseases, (2010); 10(9): 597-602.
- Ball P, Baquero F, Cars O, File T, Garau J, et al. Antibiotic therapy of community respiratory tract infections: strategies for optimal outcomes and minimized resistance emergence. Journal of Antimicrobial Chemotherapy, (2002); 49(1): 31-40.
- Abalaka M, Oyewole O, Kolawole A. Antibacterial activities of Azadirachta indica against some bacterial pathogens. Advances in life Sciences, (2012); 2(2): 5-8.
- Abalaka M, Adeyemo S, Okolo M, Damisa D. Antifungal activity of Gomphrena celosioides (soft khaki weed) on selected fungal isolates. Journal of Current Research in Science, (2013); 1(2): 66-70.
- Abalaka M, Daniyan S, Mann A. Evaluation of the antimicrobial activities of two Ziziphus species (Ziziphus mauritiana L. and Ziziphus spinachristi L.) on some microbial pathogens. African Journal of Pharmacy and Pharmacology, (2010); 4(4): 135-139.
- Abalaka M, Daniyan S, Adeyemo S, Damisa D. The Antibacterial Efficacy of Gold Nanoparticles Derived from Gomphrena celosioides and Prunus amygdalus (Almond) Leaves on Selected Bacterial Pathogens. International Journal of Biological, Food, Veterinary and Agricultural Engineering, (2014); 8(4): 348-350.
- Zahir AA, Bagavan A, Kamaraj C, Elango G, Rahuman AA. Efficacy of plant-mediated synthesized silver nanoparticles against Sitophilus oryzae. Journal of Biopesticides, (2012); 288(Suppl 5): 95-102.
- Shameli K, Yunus W, Zin WM, Ibrahim NA, Darroudi M. Synthesis and characterization of silver/clay/starch bionanocomposites by green method. Australian Journal of Basic and Applied Sciences, (2010); 4(7): 2158-2165.
- Baddam R, Kumar N, Thong K-L, Ngoi S-T, Teh CSJ, et al. Genetic fine structure of a Salmonella enterica serovar Typhi strain associated with the 2005 outbreak of typhoid fever in Kelantan, Malaysia. Journal of Bacteriology, (2012); 194(13): 3565-3566.
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life sciences, (2005); 78(5): 431-441.
- Adegboye M, Akinpelu D, Okoh A. The bioactive and phytochemical properties of Garcinia kola (Heckel) seed extract on some pathogens. African Journal of Biotechnology, (2008); 7(21).
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerging Infectious Diseases, (2001); 7(2): 178-182.
- Zaidan M, Noor Rain A, Badrul A, Adlin A, Norazah A, et al. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Tropical Biomedicine, (2005); 22(2): 165-170.
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, et al. Diversity of the human intestinal microbial flora. Science, (2005); 308(5728): 1635-1638.
- Lee EN, Griep MH, Karna SP (2011) Synthesis of Gold and Silver Nanoparticles and Characterization of Structural, Optical, and Electronic Properties. DTIC Document. http://www.arl.army.mil/arlreports/2011/ARL-TR-5763.pdf
- Yazid H, Adnan R, Hamid SA, Farrukh MA. Synthesis and characterization of gold nanoparticles supported on zinc oxide via the deposition-precipitation method. Turkish Journal of Chemistry, (2010); 34(4): 639-650.
- Itah A, Essien J. Growth profile and hydrocarbonoclastic potential of microorganisms isolated from tarballs in the Bight of Bonny, Nigeria. World Journal of Microbiology and Biotechnology, (2005); 21(6): 1317-1322.
- Shameli K, Ahmad MB, Jazayeri SD, Shabanzadeh P, Sangpour P, et al. Investigation of antibacterial properties silver nanoparticles prepared via green method. Chemistry Central Journal, (2012); 6(1): 73.
- Kluytmans J, Van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clinical Microbiology Reviews, (1997); 10(3): 505-520.
- Kemp MM, Kumar A, Mousa S, Park T-J, Ajayan P, et al. Synthesis of gold and silver nanoparticles stabilized with glycosaminoglycans having distinctive biological activities. Biomacromolecules, (2009); 10(3): 589-595.
- Nikbakht M, Yahyaei B, Pourali P. Green Synthesis, Characterization and Antibacterial Activity of Silver Nanoparticles Using Fruit Aqueous and Methanolic Extracts of Berberis vulgaris and Ziziphus vulgaris. Journal of Pure and Applied Microbiology, 9(1): 349-355.