Full Length Research Article
Characterization of cypermethrin degrading bacteria: A hidden micro flora for biogeochemical cycling of xenobiotics
Farkhanda Jabeen1, Mukhtar Ahmed*1,2, Fayyaz Ahmed2, Muhammad Bilal Sarwar2, Sidra Akhtar2, Ahmad Ali Shahid2
Adv. life sci., vol. 4, no. 3, pp. 97-107, May 2017
*- Corresponding Author: Mukhtar Ahmed (Email: mukhtar.ahmed@cemb.edu.pk)
Authors' Affiliations
2- Centre of Excellence in Molecular Biology (CEMB), 87-West Canal Bank Thokar Niaz Baig, University of the Punjab Lahore – Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Cypermethrin is a Synthetic Pyrethroid (SP) having widespread applications in agriculture and industrial sector especially in sheep dip formulations and tanneries. Rhizoremediation offers a sustainable, environment-friendly and cost-effective means to carry out remediation of contaminated soils.
Methods: Six bacterial strains were screened out and characterized at various doses of cypermethrin, heavy metal salts and antibiotics. The optimum growth conditions were determined for these bacterial isolates. The degradation of cypermethrin was confirmed through the growth of bacteria on minimal media (BHB) with cypermethrin and thin layer chromatographic analysis; retention factor values (Rf) were calculated and compared with standard Rf values.
Results: Growth curve experiments revealed that three bacterial isolates were able to grow in the presence of cypermethrin. Tolerance to the high concentration of heavy metal salts (300µgmL-1) and resistance towards different antibiotics was observed in all three bacterial isolates indicating a positive correlation between pesticide degradation and tolerance to metals and antibiotics. Bacterial strains A-C1 and B-B2 were identified as Xanthomonas maltophilia and B-C2 as Acinetobacter sp. Cypermethrin degradation occurred concomitant with bacterial growth reaching an optical density (OD600) up to 0.869.
Conclusion: Microbes present in rhizosphere have potential to mineralize the pesticides. A significant biodegradation of the cypermethrin was observed based on above mentioned lab parameters. These results paved the way for designing a multi-resistant bacterium that can be used to reverse the altered environment.
Key words: Rhizo-remediation, Synthetic Pyrethroids, Cypermethrin, Enrichment, Tannery Solid Waste
Introduction
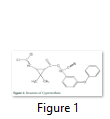
Synthetic Pyrethroids (SPs) have been used for over two decades, accounts for 25% of worldwide insecticide market [1, 2]. Cypermethrin is common and commercially available SP with widespread applications in agriculture, forestry, horticulture and urban regions to control insects and pests of cotton, fruit and vegetables since 1980s [3, 4]. It is also used to control the pests in stores, warehouses, industrial buildings, termites in houses, laboratories, treatment of ectoparasitic infestations of cattle and other livestock, food processing plants, to kill cockroaches, fleas and insects on cotton and lettuce. Chemical name of cypermethrin is (RS)-ý-cyano-3-phenoxybenzyl (1RS)-cis, trans-3- (2, 2-dichlorovinyl)-1,1-dimethylcyclopropane carboxylate (Fig. 1) and molecular weight is 415. It is distributed under different trade names by different companies.
Cypermethrin application is mostly through ground and aerial sprays. It is toxic to aquatic environment with concentration as low as 10μg/L [5-7]. Depending upon physico-chemical properties of soil its persistence in environment varies from 14.6 to 76.2 days (half-life). Due to non-polar nature and low water solubility cypermethrin readily adsorbed on to soil surface [4]. Owing to the unawareness of the toxicity of these pollutants among the farm workers and lack of proper monitoring/enforcement of law, the pesticides spread into environment and result in acute and chronic effects on human health. Prolong exposure on human body creates many health problems including cancers, birth defect, disturbance of normal metabolic process, skin allergy, eye irritation and also effects on CNS [3,8,9]. It also effects on beneficial organisms and causes pollution of soil, water and atmosphere of ecosystem [10]. Long time and persistent exposure of these harmful chemicals result in accumulation in animal bodies through food chain and then circulate throughout the body, deposit in adipose tissues and excreted during lactation. Pesticides residues have been detected in human blood [11-13], cattle milk [14] and fruits [15]. Cypermethrin effects on vertebrates and invertebrates nervous system by producing hyper excitable state and damaging the voltage dependent sodium channels. As a result of this, sodium channels remain open for longer period than normal [16].
Different means of pesticide degradation includes microbial, chemical or photo-degradation. Microbial degradation occurs by microorganisms that uses these substances as an energy source. The potential of indigenous microorganisms to degrade such pollutants can be used to clean up xenobiotics and represents a potential solution to such environmental problems [17, 18]. The microbes have capability to make use of all other pyrethroids present in water and soil as their carbon and energy source. Biodegradation in organic compounds lead to conversion of carbon, nitrogen, sulphur, phosphorus and other elements in the original compounds to inorganic products [19, 20]. Bacteria can live in a variety of habitat on earth due to their high metabolic diversity. Some important pyrethroid degrading bacteria include Pseudomonas fluorescens [21], Bacillus cereus [22], Burkholderia picketti, Erwinia carotovora, Vibrio hollisae [23] and have been isolated from soil and rivers contaminated with pesticides. Similarly Murugesan et al. isolated five potential cypermethrin degrading bacterial strains together with Bacillus sp. from Brinjal (Solanum melangena) cultivated soil [24]. Wheat rhizoplane is rich with potential microbes like Bacillus pumilus, Pseudomonas fluorescence, Bacillus subtillus, Streptomyces spp. and Xanthomonas [25]. Biodegrading bacteria are widely distributed in marine, paddy water and soil habitats [26, 27]. The plant rhizosphere is also a good habitat for bacteria that may have auxiliary characteristics such as nitrogen fixation, mineral utilization or plant growth regulator production in addition to biodegradation capability. The existence of assisted traits suggests the agronomic and environmental impact of these microbes [4, 28]. These bacteria can be isolated from pesticide contaminated sites by different methods. Pesticide degrading bacteria were isolated using rapid screening method as described previously [29]. However, the most common and reliable technique for isolation is enrichment technique [30, 31]. Enrichment involves the continuous stress of a particular pollutant for the adaptation of microbes. Different time periods are used for enrichment, Maloney et al. applied up to eight weeks and subculture every two weeks thereafter [32].
The present study was conducted with an aim to isolate local strains of cypermethrin degrading bacteria and to evaluate their characteristic with purpose of bioremediation of pesticide contaminated soils and waste water. These isolates have the potential to clean up the environment from such pollutants. These indigenous bacterial strains may be conveniently used in microbe based system and will provide a baseline data for future studies in biodegradation. The appealing trait of these isolates is their mutual association with plants roots that made these strains ecological triumph for bioremediation of pollutants. This preliminary study will also be helpful for the municipal authorities in setting up relevant management policies.
Methods
Soil sample collection
Soil samples used for isolation and screening of bacterial strains were taken from 0-12cm depth from rhizosphere of different plants (Pisum sativum, Pennisetum pedicillatum, Chenopodium album, Triticum aestivum) growing at tannery solid waste District Kasur (31° 6' 56" N, 74° 26' 48" E) Punjab, Pakistan (Table 1). In tanneries pesticides have been continuously applied for multiple decades for processing of hides and industrial effluents are thrown in field. For isolation of cypermethrin degrading bacteria samples were placed into sterilin autoclaved polyethylene bags and further processed in the laboratory.
Isolation of cypermethrin degrading bacteria by enrichment and screening
Enrichment culture technique was used for isolation of bacteria in soil collected from pesticide contaminated sites [33]. Bushnell-Haas Broth (BHB) minimal media g L-1, [K2HPO4, 1.0; KH2PO4, 1.0; NH4NO3, 1.0; CaCl2, 0.02; MgSO4, 0.2; FeCl3, 0.05, adjusted to pH 7] was used [34]. Initial cultivation to obtain isolated colonies LB media [trypton, 10; Yeast extract, 5; NaCl, 5; and agar, 15 g L-1, pH 7.0] was used. Enrichment and isolation of pure culture was performed by the methods as described by previous studies [31, 35, 36]. Cypermethrin stock solution 10% (w/v) for all the experiments was prepared in acetone. Six different strains of bacteria (A-C1, A-C2, B-B2, B-C2, D-C1, and D-C2) were isolated from four different soil samples.
Characterization and identification of cypermethrin biodegrading microorganisms
Morphological and biochemical characterization was performed according to earlier protocols [37, 38]. Gram-negative bacteria were identified through Microbact Gram-negative 24E system kit MB1130. Colonies suspended in sterile normal saline (0.85%), suspensions were added to wells and reading of reaction in each well was taken after 24 and 48 hours incubation at 37oC. The results were then interpreted for bacterial identification according to manufacturer’s instructions and also using results of conventional biochemical tests [39].
Antibiotic sensitivity and metal tolerance
Response of various antibiotics (Ampicillin, Chloramphenicol, Carbencillin, Fusidic acid, Linomycin and Clarithromycin) through antibiotics discs (Oxide, Wade Road, UK) and heavy metals solutions (CuSO4, MnSO4, ZnSO4, NiCl2, CoSO4, Na2SO4, K2Cr2O7) with 100µgmL-1 and 300µgmL-1 were checked by incubating plates of L-agar supplemented with antibiotic discs and salt solution at 30oC for 24-48 hours [40].
Growth and influence of physio-biochemical parameters
Microbial growth in LB and BHB broth with cypermethrin monitored by incubating culture at 30oC and 2 RCF on rotary shaker. The OD was taken at 600nm immediately after inoculation and with intervals 2, 4, 6, 8, 10, 12, 14 and 15 hours. The isolates were optimized for their physical growth factors like temperature, pH, inoculum size and Minimal Inhibitory Concentration (MIC) of cypermethrinin in LB-broth and BHB by taking mean OD at 600nm in triplicates. The optimal range of these factors determined in this study is, temperature: 25-56oC, pH: 5-10, inoculum volume: 125, 250 and 500μL and MIC: 50-250μg/mL.
Biodegradation study
Cypermethrin degradation studies in flask
Biodegradation study of cypermethrin accomplished using BHB media in 100mL Erlenmeyer flasks. A 50mL BHB with 100µgmL-1 cypermethrin inoculated with 10% over night bacterial culture. Flasks were placed at ambient conditions on orbital shaker OS-752 (OPTMA Japan) at 2 RCF for one week. Two control flasks one with bacterial culture but without cypermethrin, other with cypermethrin and without bacterial culture maintained under similar conditions to check the loss of cypermethrin, if any either due to chemical hydrolysis or dissipation. The OD of the culture was determined spectrophotometrically at 600nm.
Analytical procedure for residues extraction
Cypermethrin residues were extracted by mixing ethyl acetate in sample culture (1:1 by volume) in 50mL conical flask by vigorous shaking at 2 RCF for 1 hour. The organic phase was carefully eluted by passing through anhydrous sodium sulphate (Na2SO4) column (6cm), made in pasture pipette stopped with glass wool to remove water contents. The organic solvent ethyl acetate was allowed to dry on rotary evaporator and dried sample was dissolved in methanol (Merck HPLC grade), then applied to TLC plate [41, 42].
Thin Layer Chromatography (TLC)
Readymade pre coated silica gel plates (silica gel 60 F254 0.25mm thicknesses, 20×20 cm Merck Ltd. Germany) used for TLC of cypermethrin [43, 44]. The sample volume of 5µL was spotted on silica gel TLC plates at 1 cm apart with the help of calibrated micropipette along with same volume of pure cypermethrin as standard to identify the components of sample. The plates were placed in a glass tank containing Benzene: Ethyl acetate (6:1 by volume) solvent system as mobile phase. The plates were kept in ascending direction, when solvent front reached the reference line after traveling 75% distance, the plates removed and distance travelled by solvent front was marked instantly with lead pencil and extra solvent was allowed to evaporate in fume hood at room temperature. Then plates were exposed to U.V at 245nm for about 20 minutes. The spots marked and Rf values were calculated.
Results
Isolation of cypermethrin degrading bacteria
Six different strains of bacteria were isolated from soil samples by streaking on agar plates. The bacterial colonies of these six strains (A-C1, A-C2, B-B2, B-C2, D-C1, and D-C2) streaked on sterilized minimal media (BHB) with cypermethrin 100 µgmL-1, the strains that showed growth at this concentration were re-streaked on higher concentration of cypermethrin up to 200 µgmL-1. Maximum growth showing strains further streaked on plates of BHB at 250µgmL-1 cypermethrin. Only three strains A-C1, B-B2, and B-C2 survived at 250 µgmL-1 dose of cypermethrin were selected for further cypermethrin degradation studies.
Morphological and biochemical characterization of bacteria
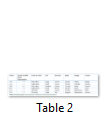
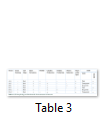
Individual colonies were characterized on the bases of morphology and cultural characteristics results shown in Table 2. Gram staining, spore staining, and motility, catalase, oxidase and nitrate reduction tests were performed. All best growing strains (A-C1, B-B2, and B-C2) were found gram negative (Table 3).
Identification of cypermethrin degrader through microbact 24E
Three bacterial strains exhibit best growth on BHB with cypermethrin were gram negative and further characterized through Microbact 24Ekit (MB1130 Oxoid, Wade Road, UK) (Table 4), out of these three, two (A-C1 and B-B2) were identified as Xanthomonas maltophilia and remaining one (B-C2) as Acinetobacter sp.
Antibiotic sensitivity and metal tolerance
Growth response of bacteria to antibiotics was checked by applying antibiotic discs on agar plates. All three strains were resistant to Ampicillin (AMP), Carbenicillin (CAR), Linomycin (MY) and sensitive to Chloramphenicol (C). However they exhibited different response to Fusidic acid (FD) and Clarithromycin (CLR), Xanthomonas maltophilia A-C1 and Xanthomonas maltophilia B-B2 are sensitive to these two antibiotics but Acinetobacter sp. B-C2 is resistant to Clarithromycin (CLR). Bacteria streaked on agar plates
supplemented with heavy metal salts at concentration of 100, 300μgmL-1 and placed at 37oC for 24 to 48 hours (Fig. 2). It was noteworthy that three strains were resistant to all heavy metal salts used in experiment except Xanthomonas sp. B-B2 that is sensitive to ZnSO4 and K2CrO7 salts at 300μgmL-1.
Physio-biochemical conditions
Optimization of conditions for cypermethrin degradation
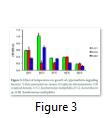
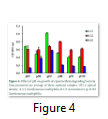
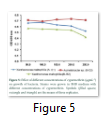
Physical growth factors have significant role, they influence microbial growth and pesticide degradation efficiency by bacteria. Temperature is highly variable in different areas, so it is considered as significant factor in cypermethrin degradation. The data reported in this study indicates that Xanthomonas maltophilia A-C1 and Xanthomonas maltophilia strain B-B2 can grow and retain their degradation capability in wide range of temperature (25-56oC) and pH (5-10) with optimum degradation at 30oC and neutral pH (7.0). These results are consistent with findings of Sing and Seth that Pseudomonas strain can survive at pH 5.5 to 9.5 with best growth at 7.0 [45]. Whereas optimum temperature and pH for Acinetobacter B-C2 were at 30oC, pH 8.0 (Fig. 3, 4). In another study conducted it was investigated that significant removal of cypermethrin occurred in biosimulator when operated at 28-30oC temperature using Pseudomonas strain [46]. In biological treatment of sewage, tolerable limit of pH in activated sludge ranged from 6.0 to 9.0 [47]. The strain Xanthomonas maltophilia A-C1 showed maximum growth at inoculums volume 250µL and MIC 100 µgmL-1 and for Xanthomonas maltophilia B-B2 strain 500µL and 200µgmL-1. In case of Acinetobacter B-C2 inoculum volume has no significant effect but MIC is 200µgmL-1 (Fig. 5). This indicates that the optimum dose of cypermethrin for these three bacterial isolates is different however high concentration is not toxic to all three, but their ability to mineralize pesticide is reduced, that may result in extended lag phase.
Bacterial growth in LB and BHB broth
Growth results depict raise of cell biomass in both (LB and BHB) media up to 12 hours as OD found to be increased (OD600nm up to 1.8), constant or decreased as the incubation progressed towards end of experiment that might be due to consumption of all available nutrients and depletion of oxygen (Fig. 6). Studies have investigated that population density increase in media is indication of degradation process and increase of cell mass [48].
Biodegradation study
In present study growth experiments conducted by using specific amount of cypermethrin to know whether these isolates are able to survive in presence of added cypermethrin. Relative growth response of the strains in presence of two different concentrations of cypermethrin (100µgmL-1, 250µgmL-1) along with control was determined. The ODs determined spectrophotometrically at 600nm for Xanthomonas maltophilia A-C1, Xanthomonas maltophilia B-B2 and Acinetobacter B-C2 were 0.709, 0.789, and 0.869 respectively in experimental flask however no growth was observed in control flasks. The increase in OD represents rise in population density of bacterial isolates in experimental flask compared to control as the degradation continued. The increase of population density determined by increase of OD was clue of metabolic activity reflected in the form of increased cell count. Cypermethrin degradation by bacterial strains was also confirmed by TLC.
Cypermethrin standard was spotted along with extracts from two different bacterial cultures Xanthomonas maltophilia A-C1 and Xanthomonas maltophilia B-B2 on TLC plate. The Rf value obtained for standard was 0.85 while the Rf value from A-C1 was 0.83 in lane no. 2 and B-B2 was 0.83 in lane 3 (chromatogram A). Sample from un-inoculated flask was spotted on lane 1 with Rf 0.85 and in lane 2 spot from Acinetobacter B-C2 with Rf 0.85, while in lane 3 sample from flask without cypermethrin was applied and no spot appeared (chromatogram B). The intensity of spot color in A-C1 is lighter as compared to spots from B-B2, B-C2 and standard cypermethrin that depict greater rate of cypermethrin degradation by Xanthomonas maltophilia A-C1 as compared to others. The Rf values of all the tested samples are in close agreement with that of standard one. The TLC plates with spotted experimental and controlled samples are presented in Fig. 7. No dissipation of cypermethrin was observed in agitated control flask. This indicates that there was no degradation of cypermethrin with abiotic factors.
Tables & Figures
Discussion
SPs have made a great impact on our economy both directly and indirectly by controlling insects and pests in agriculture, houses, and gardens and also improving yield. In developing countries, use of pesticides is increasing with the aim to accomplish higher agriculture production, to meet the food requirements of growing population and to enable the farmers to reap the benefits from agricultural investments. Studies have investigated that SPs might have teratogenic, mutagenic, neurotoxic and endocrine disruptive effects [49]. Cypermethrin belongs to fourth generation bio-pesticides compound of SPs which is insoluble in water. It has strong affinity to adsorb to soil particles producing soil surface contamination [50], hence difficult to remove by conventional treatment methods. Biodegradation of SPs including cypermethrin has emerged as an important paradigm in environment toxicology due to which its removal from environment is obligatory. The biological method of remediation is favored as compared to physical and chemical methods owing to its cost effectiveness and environmental friendliness.
The study dealt with the isolation, identification and degradation efficiency judgement of bacterial strains having ability to degrade cypermethrin as carbon source. Three active bacterial isolates Xanthomonas maltophilia A-C1, Xanthomonas maltophilia B-B2 and Acinetobacter B-C2 exhibited growth on different cypermethrin concentrations reflecting their ability to use it as sole carbon and energy source. These are gram negative, rod shape, motile, oxidase negative, yellow pigmented. These results are closely related to the findings [42, 51]. Similarly Tallur et al. [3] isolated Micrococcus sp. strain CPN 1 from soil capable of using pyrethroids like cypermethrin, deltamethrin and permethrin as carbon source. Other strains Pseudomonas sp. and Serratia sp were isolated from soil and sheep dip that were capable of utilizing cypermethrin and flumethrin as energy source [30, 34]. The results of enrichment were three cypermethrin degrading strains that can survive at high cypermethrin dose (250µgmL-1). However, the maximum growth of each strain was different at different pesticide concentration i.e. Xanthomonas maltophilia A-C1 (100µgmL-1), Xanthomonas maltophilia B-B2 (50µgmL-1) and for Acinetobacter B-C2 (200µgmL-1) (Fig. 5). From biomass determined turbidimetrically at 600 nm it was investigated that the number of organisms at high cypermethrin concentration (250µgmL-1) decreases but there is no growth inhibition. The plausible explanation might be that microbes require an acclimation period to induce synthesis of essential enzymes for degradation. Another reason for low growth in presence of high amount of cypermethrin may be limited supply of dissolved oxygen (DO) [52]. Previous study also support that Acinetobacter sp. can utilize wide range of toxicants as biphenyl and chlorinated biphenyl as sole carbon and energy source [53]. Another important feature which is worth mentioning is that bacterial strains isolated in current study exhibited tolerance to heavy metal salts and antibiotics. These metals and antibiotics are present in soil as in case of tannery solid waste and they are detrimental to health, kills the majority of the micro flora and also inhibit biodegradation of organic pollutants in contaminated sites. This observation is quite agreed with previous finding [35, 40, 54].
Previous studies revealed that environmental factors such as temperature, pH and inoculums size have important role in biodegradation process of xenobiotics by microbes [55-58]. Our findings indicate that the strains engaged in degrading Cypermethrin in a range of temperature 25-56oC, pH 5-10 and inoculums volume 125, 250, 500µL. This is the significant characteristic of these strains to be employed for bioremediation of variable environment. The optimum temperature for three strains was 30oC and pH 7-8. Initial inoculum size 250µL was sufficient for Xanthomonas maltophilia A-C1 to degrade cypermethrin but Xanthomonas maltophilia B-B2 and Acinetobacter B-C2 showed better degradation at 500µL. However these strains also degraded cypermethrin at minimum inoculums volume, provided the culture had been incubated for long time. Small inoculums size lead to extended lag phase prior to rapid degradation took place. During degradation of xenobiotics an acclimation period reveals the time necessary for duplication of small, active population of microbes to a definite level sufficient to quickly degrade these pollutants [59].
The retention factor (Rf) value of pesticide can never be changed by any environmental factor or with passage of time. The Rf values of all tested samples calculated from TLC plate are mentioned in Fig.7. The results are in close agreement with those obtained by Chen and Wang [60]. They investigated Rf values of different pesticides using different solvent systems. The values obtained from Xanthomonas maltophilia A-C1, B-B2 and Acinetobacter B-C2 are 0.83 and 0.85 respectively, that are almost close to 0.80 which is 3-phenoxybenzoic acid, a metabolite of cypermethrin and trans Beta-cypermethrin (0.81) [61].
OG2 strain Stenotrophomonas maltophilia bacteria was isolated from body micro-flora of cockroaches that can convert alpha cypermethrin to 3-phenoxybenzoic acid and other products [42]. In another study, Stenotrophomonas maltophilia MHF ENV 22 was isolated from rhizosphere of Pennisetum pedicellatum that degrade cypermethrin in soil and produce 3-phenoxybenzoic acid as an intermediate product [41]. In our case Acinetobacter B-C2 is isolated from rhizosphere of Pennisetum pedicellatum that metabolize cypermethrin. The degradation of pyrethroids including cypermethrin by Acinetobacter has also been reported by Jin et al. [61]. Xenobiotic degradation by genus Acinetobacter and Brevibacillus have been reported in previous studies [62-64]. From above findings we can speculate that the pathway of cypermethrin degradation by our indigenous strains is same as described by Tallur et al. [3].
In present study three bacterial strains Xanthomonas maltophilia A-C1, B-B2 and Acinetobacter B-C2 from rhizosphere of Pisum sativum, Pennisetum pedicellatum that may participate in efficient cypermethrin degradation in contaminated agricultural soil, resist high doses of heavy metals together with different antibiotics were isolated. The organisms with combination of antibiotic and heavy-metal resistance would be useful for bioremediation of polluted environments. Such organisms would be able to compete well with antibiotic-producing flora in the polluted environment. Degradation of cypermethrin occurred at range of pH and temperature, this feature enables them to play important role in cypermethrin biogeocycle. Future investigations are necessary to explore the aptitude of isolates that harbor the ability to degrade pesticides when introduced into highly competitive soil environment. The rhizospheric dissipation capability of these plants along with microbes make the successful implementation of this green technology for decontamination and remediation of sites polluted with pesticides.
Acknowledgment
The authors are thankful to Higher Education Commission (HEC) Islamabad for providing financial support to complete this work.
References
- Gilbert LI, Gill SS. Insect control: biological and synthetic agents.. 2010; Academic Press.
- Liu W, Gan J, Lee S, Werner I. Isomer selectivity in aquatic toxicity and biodegradation of bifenthrin and permethrin. Environmental Toxicology and Chemistry, (2005); 24(8): 1861-1866.
- Tallur PN, Megadi VB, Ninnekar HZ. Biodegradation of cypermethrin by Micrococcus sp. strain CPN 1. Biodegradation, (2008); 19(1): 77-82.
- Akbar S, Sultan S, Kertesz M. Determination of cypermethrin degradation potential of soil bacteria along with plant growth-promoting characteristics. Current Microbiology, (2015); 70(1): 75-84.
- Vinodhini R, Narayanan M. Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). International Journal of Environmental Science & Technology, (2008); 5(2): 179-182.
- Virtue W, Clayton J. Sheep dip chemicals and water pollution. Science of the total environment, (1997); 194207-217.
- Pearce F. Sheep dips poison river life. 1997. New Scientist Publ Expediting Inc 200 Meacham Ave, Elmont, NY 11003.
- Hoof Fv, Wiele Pv, Acobas F, Guinamant J-L, Bruchet A, et al. Multiresidue determination of pesticides in drinking and related waters by solid-phase extraction and liquid chromatography with ultraviolet detection: Interlaboratory study. Journal of AOAC International, (2002); 85(2): 375-383.
- Kakko I, Toimela T, Tähti H. Oestradiol potentiates the effects of certain pyrethroid compounds in the MCF7 human breast carcinoma cell line. Alternatives to laboratory animals: ATLA, (2004); 32(4): 383-390.
- Leistra M, Smelt JH, Weststrate JH, van den Berg F, Aalderink R. Volatilization of the pesticides chlorpyrifos and fenpropimorph from a potato crop. Environmental Science & Technology, (2006); 40(1): 96-102.
- Park MJ, In SW, Lee SK, Choi WK, Park YS, et al. Postmortem blood concentrations of organophosphorus pesticides. Forensic science international, (2009); 184(1): 28-31.
- Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. Journal of Biological Chemistry, (2004); 279(47): 49064-49073.
- Soomro A, Seehar G, Bhanger M, Channa N. Pesticides in the blood samples of spray-workers at agriculture environment: The toxicological evaluation. Pakistan Journal of Analytical & Environmental Chemistry, (2008); 9(1): 32-37.
- Gazzotti T, Sticca P, Zironi E, Lugoboni B, Serraino A, et al. Determination of 15 organophosphorus pesticides in Italian raw milk. Bulletin of environmental contamination and toxicology, (2009); 82(2): 251-254.
- Ahmad M, Hussain A, Ahmad R, Asi M. Determination of Cypermethrin and Dithiocabamate Residues in Muskmelons. Science International-Lahore, (1994); 6(4): 355-360.
- Vijverberg HP, vanden Bercken J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Critical Reviews in Toxicology, (1990); 21(2): 105-126.
- Widada J, Nojiri H, Omori T. Recent developments in molecular techniques for identification and monitoring of xenobiotic-degrading bacteria and their catabolic genes in bioremediation. Applied Microbiology and Biotechnology, (2002); 60(1-2): 45-59.
- Bosma T, Damborský J, Stucki G, Janssen DB. Biodegradation of 1, 2, 3-trichloropropane through directed evolution and heterologous expression of a haloalkane dehalogenase gene. Applied and Environmental Microbiology, (2002); 68(7): 3582-3587.
- Morrica P, Giordano A, Seccia S, Ungaro F, Ventriglia M. Degradation of imazosulfuron in soil. Pest Management Science, (2001); 57(4): 360-365.
- Esteve-Núñez A, Caballero A, Ramos JL. Biological degradation of 2, 4, 6-trinitrotoluene. Microbiology and Molecular Biology Reviews, (2001); 65(3): 335-352.
- Grant R, Daniell T, Betts W. Isolation and identification of synthetic pyrethroid‐degrading bacteria. Journal of Applied Microbiology, (2002); 92(3): 534-540.
- Maloney S, Maule A, Smith AR. Purification and preliminary characterization of permethrinase from a pyrethroid-transforming strain of Bacillus cereus. Applied and Environmental Microbiology, (1993); 59(7): 2007-2013.
- Lee S, Gan J, Kim JS, Kabashima JN, Crowley DE. Microbial transformation of pyrethroid insecticides in aqueous and sediment phases. Environmental Toxicology and Chemistry, (2004); 23(1): 1-6.
- Murugesan A, Jeyasanthi T, Maheswari S. Isolation and characterization of cypermethrin utilizing bacteria from Brinjal cultivated soil. African Journal of Microbiology Research, (2010); 4(1): 010-013.
- Juhnke ME, Mathre D, Sands D. Identification and characterization of rhizosphere-competent bacteria of wheat. Applied and Environmental Microbiology, (1987); 53(12): 2793-2799.
- Calero S, Fomsgaard I, Lacayo ML, Martínez V, Rugama R. Preliminary study of 15 organochlorine pesticides in Lake Xolotlan, Nicaragua. Chemosphere, (1992); 24(9): 1413-1419.
- Thao VD, Kawano M, Tatsukawa R. Persistent organochlorine residues in soils from tropical and sub-tropical Asian countries. Environmental Pollution, (1993); 81(1): 61-71.
- Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ. Influence of plant growth promoting bacteria and Cr 6+ on the growth of Indian mustard. Chemosphere, (2006); 62(5): 741-748.
- Smejkal C, Vallaeys T, Burton S, Lappin‐Scott H. A rapid method to screen degradation ability in chlorophenoxyalkanoic acid herbicide‐degrading bacteria. Letters in Applied Microbiology, (2001); 32(4): 273-277.
- Jilani S, Altaf Khan M. Isolation, characterization and growth response of pesticides degrading bacteria. Journal of Biological Sciences, (2004); 4(1): 15-20.
- Siddique T, Okeke BC, Arshad M, Frankenberger WT. Enrichment and isolation of endosulfan-degrading microorganisms. Journal of Environmental Quality, (2003); 32(1): 47-54.
- Maloney S, Maule A, Smith AR. Microbial transformation of the pyrethroid insecticides: permethrin, deltamethrin, fastac, fenvalerate, and fluvalinate. Applied and Environmental Microbiology, (1988); 54(11): 2874-2876.
- El-Fantroussi S, Verstraete W, Top EM. Enrichment and molecular characterization of a bacterial culture that degrades methoxy-methyl urea herbicides and their aniline derivatives. Applied and Environmental Microbiology, (2001); 67(10): 4943.
- Grant R, Betts W. Mineral and carbon usage of two synthetic pyrethroid degrading bacterial isolates. Journal of Applied Microbiology, (2004); 97(3): 656-662.
- Ahmed M, Jabeen F, Ali M, Ahmad Z, Ahmed F, et al. Analysis of Bifenthrin Degrading Bacteria from Rhizosphere of Plants Growing at Tannery Solid Waste. American Journal of Plant Sciences, (2015); 6(13): 2042.
- Kumar K, Devi SS, Krishnamurthi K, Kanade GS, Chakrabarti T. Enrichment and isolation of endosulfan degrading and detoxifying bacteria. Chemosphere, (2007); 68(2): 317-322.
- Cappuccino J, Sherman N. Microbiology, Laboratory Manual. 2004. Person education, USA.
- Gerhardt P. Methods for general and molecular bacteriology. 1994. American Society for Microbiology, USA.
- Williams S, Sharpe M, Holt J. Bergey’s manual of determinative bacteriology. Baltimore: Williams and Wilkins Ji, GD, Sun, TH, Ni, JR, & Tong, JJ (2009) Anaerobic baffled reactor (ABR) for treating heavy oil produced water with high concentrations of salt and poor nutrient. Biore-source Technology, (1994); 100(3): 11081114.
- Naphade SR, Durve AA, Bhot M, Varghese J, Chandra N. Isolation, characterization and identification of pesticide tolerating bacteria from garden soil. European Journal Of Experimental Biology, (2012); 2(5): 1943-1951.
- Dubey KK, Fulekar M. Investigation of potential rhizospheric isolate for cypermethrin degradation. 3 Biotech, (2013); 3(1): 33-43.
- Gür Ö, Özdal M, Algur ÖF. Biodegradation of the synthetic pyrethroid insecticide α-cypermethrin by Stenotrophomonas maltophilia OG2. Turkish Journal of Biology, (2014); 38(5): 684-689.
- Oi M. Time-dependent sorption of imidacloprid in two different soils. Journal of Agricultural and Food Chemistry, (1999); 47(1): 327-332.
- Manzoor F, Asma S, Fazal S, Abbas M, Noor M. Estimation of degradation of different termiticides under field conditions using TLC method. Science Technology and Development, (2012); 31(2): 128-132.
- Singh AK, Seth P. Degradation of malathion by microorganisms isolated from industrial effluents. Bulletin of Environmental Contamination and Toxicology, (1989); 43(1): 28-35.
- Jilani S. Comparative assessment of growth and biodegradation potential of soil isolate in the presence of pesticides. Saudi Journal of Biological Sciences, (2013); 20(3): 257-264.
- Hänel K, Hemmings B Biological treatment of sewage by the activated sludge process.. 1988; Ellis Horwood Chichester.
- Na K-s, Kuroda A, Takiguchi N, Ikeda T, Ohtake H, et al. Isolation and characterization of benzene-tolerant Rhodococcus opacus strains. Journal of Bioscience And Bioengineering, (2005); 99(4): 378-382.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environmental Health Perspectives, (2005); 123-136.
- Casida JE. Pyrethrum flowers and pyrethroid insecticides. Environmental Health Perspectives, (1980); 34189.
- Azmy AF, Saafan AE, Essam TM, Amin MA, Ahmed SH. Biodegradation of Malathion by Acinetobacter baumannii Strain AFA Isolated from Domestic Sewage in Egypt. Biodegradation, (2015); 117465.
- Corbitt RA. Standard Handbook of Environmental Engineering. 1990; McGraw-Hill, New York, USA.
- Sabit HH, Said OA, Shamseldin AF, Elsayed K. Molecular identification of acinetobacter isolated from egyptian dumpsite as potential bacteria to degrade malathion. International Journal of Academic Research, (2011); 3(4): 84-90.
- Oyetibo GO, Ilori MO, Adebusoye SA, Obayori OS, Amund OO. Bacteria with dual resistance to elevated concentrations of heavy metals and antibiotics in Nigerian contaminated systems. Environmental Monitoring and Assessment, (2010); 168(1-4): 305-314.
- Karpouzas D, Walker A. Factors influencing the ability of Pseudomonas putida strains epI and II to degrade the organophosphate ethoprophos. Journal of Applied Microbiology, (2000); 89(1): 40-48.
- Cycoń M, Wójcik M, Piotrowska-Seget Z. Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere, (2009); 76(4): 494-501.
- Singh BK, Walker A, Wright DJ. Bioremedial potential of fenamiphos and chlorpyrifos degrading isolates: influence of different environmental conditions. Soil Biology and Biochemistry, (2006); 38(9): 2682-2693.
- Anwar S, Liaquat F, Khan QM, Khalid ZM, Iqbal S. Biodegradation of chlorpyrifos and its hydrolysis product 3, 5, 6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1. Journal of Hazardous Materials, (2009); 168(1): 400-405.
- Chen S, Alexander M. Reasons for the acclimation for 2, 4-D biodegradation in lake water. Journal of environmental quality, (1989); 18(2): 153-156.
- Chen Z-M, Wang Y-H. Chromatographic methods for the determination of pyrethrin and pyrethroid pesticide residues in crops, foods and environmental samples. Journal of Chromatography A, (1996); 754(1): 367-395.
- Jin Z, Guo Q, Zhang Z, Yan T. Biodegradation of type II pyrethroids and major degraded products by a newly isolated Acinetobacter sp. strain JN8. Canadian Journal of Microbiology, (2014); 60(8): 541-545.
- LIU F-y, HONG M-z, LIU D-m, LI Y-w, SHOU P-s, et al. Biodegradation of methyl parathion by Acinetobacter radioresistens USTB-04. Journal of Environmental Sciences, (2007); 19(10): 1257-1260.
- Reddy MS, Naresh B, Leela T, Prashanthi M, Madhusudhan NC, et al. Biodegradation of phenanthrene with biosurfactant production by a new strain of Brevibacillus sp. Bioresource Technology, (2010); 101(20): 7980-7983.
- Singh B, Kaur J, Singh K. Biodegradation of malathion by Brevibacillus sp. strain KB2 and Bacillus cereus strain PU. World Journal of Microbiology and Biotechnology, (2012); 28(3): 1133-1141.