Full Length Research Article
Optimization of sulphuric acid pre-treatment of Acacia saw dust through box-bhenken design for cellulase production by B. Subtilis
Aasiya Anjum1, Muhammad Irfan2*, Fouzia Tabbsum1, Hafiz Abdullah Shakir1, Javed Iqbal Qazi1
Adv. life sci., vol. 5, no. 1, pp. 19-24, November 2017
*- Corresponding Author: Muhammad Irfan (Email: irfan.biotechnologist@gmail.com)
Authors' Affiliations
2. Department of Biotechnology, University of Sargodha – Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Cellulases are enzymes which are capable of degrading lignocellulosic biomass. The current study is centred on optimization of dilute sulphuric acid pre-treatment of Acacia saw dust for maximizing cellulase production (CMCase and FPase). Hydrolysis or saccharification of lignocellulosic biomass is brought about by cellulases and the sugar thus released can be used for further bioethanol production.
Methods: Box- Bhenken design (BBD) was employed for optimization of pre-treatment conditions for Acacia saw dust. Three variables i.e. sulphuric acid concentration (0.6%, 0.8% and 1.0% v/v), substrate concentration (5%,10% and15%) and reaction time (4h,6h and 8h) was optimized. The pre-treated saw dust was used in the study as a substrate for producing cellulase enzyme through submerged fermentation by Bacillus subtilis (K-18).
Results: An optimum conditions i.e. (0.8% H2SO4 conc., 15% substrate conc. and 4h of reaction time) yielded highest filter paper activity (1.3617 IU/ml/min) and CMCase activity (0.7783 IU/ml/min). The suggested model was significant as revealed by F-value, coefficient of determination (R2) and P-value.
Conclusion: Results concluded that pre-treated substrate (Acacia sawdust) significantly increased cellulase production as compared to untreated substrate that could be utilized for further biofuel production.
Key words: Saw dust, Pre-treatment, Cellulase, RSM, Bacillus sp.
Introduction
Kikar (Acacia arabica) is a lignocellulosic biomass, having about 40-50% cellulose, 18% hemicelluloses and 25% lignin [1]. Cellulose, the major part of plant cell wall has plentiful existence on earth. It is cheap raw material and can be used for bioethanol production [2]. Cellulose is homopolymer, unbranched polysaccharide formed of repeated β-1, 4 glucose units associated by β-1, 4-glycosidic bonds. Hemicellulose is a heteropolymer formed of different sugars such as D-glucose, D-galactose and others. Lignin contains phenylpropane units that are linked in 3- dimensional pattern. Lignin seals cellulose and hemicellulosic component of lignocelluloses and thus impedes enzymes accessibility to cellulose. Therefore, pre-treatment is necessary for delignification and subsequent enzymatic hydrolysis process [3]. Pre-treatment process must meet the characteristics such as it should avoid cellulose and hemicellulose degradation, it should avoid inhibitors formation and overall process cost must be less [3]. Different pre-treatment methods are available like physical method, chemical method (ammonia treatment, steam explosion, ozone treatment). Among different pre-treatment methods, acid and base pre-treatment is commonly employed for treating lignocellulosic biomass. The reason is that they give high reaction rate and the process is less costly than other processes.
Lignocellulosic biomass can be hydrolysed both by chemical or enzymatic method. Enzymatic hydrolysis is carried with fungi and bacteria that split complex cellulose polymers into glucose units by hydrolytic enzymes to utilize it as a carbon source. Complete hydrolysis of cellulose is brought about by synergistic action of three enzymes: i) Endoglucanase or CMCase destroys crystalline structure of celluloses by splitting internal bond. ii) Exoglucanase (also called avicelase or cellobiohydrolase) cleaves reducing and non-reducing ends of oligomers to produce cellobiose that is a disaccharide.iii) β- Glucosidases further cleaves cellobiose to release glucose units [4].
Cellulose digesting enzymes are produced from various bacterial and fungal species. Aspergillus, Phanerochaete chrysosporium, Penicillium, Trichoderma and Fusarium are some fungal species that possess high cellulase activity [5, 6]. Among bacterial genera, Bacillus, Clostridium, Cellulomonas, Pseudomonas possess high cellulase potential [7]. Cellulases have various applications: being used in pulp and paper industry, animal feed, textile, food, brewing, fuel and waste treatment [8].
Response surface methodology (RSM) is a mathematical and statistical mean for checking the fitness value of a variable for optimization of response in multivariable system. In RSM, association between input parameters and response can be studied. It is used whenever several variables are affecting response [9].The current study aims at optimization of dilute sulphuric acid pre-treatment of saw dust for producing cellulase enzyme through Bacillus subtilis K-18 through submerged fermentation.
Methods
Microorganism
Bacillus subtilis K-18 was procured from Microbial Biotechnology Laboratory, Department of Zoology, University of the Punjab. The culture was maintained on nutrient agar slants and then it was employed for producing cellulase enzyme through submerged fermentation.
Pre-treatment process of Saw Dust
Acacia saw dust was collected from local market of Ichra Lahore Pakistan. The saw dust was treated with varying concentration of dilute sulphuric acid according to Box-Behnken Design as described in our earlier reports [10]. Briefly, the specific amount of substrate was mixed with 100ml solution of specific concentration of sulphuric acid and left at room temperature for various time period as per design. After the termination of time period, the slurry was filtered and solid biomass was washed up to neutral pH and dried for further processing.
Enzyme Production
CMCase and FPase enzyme was produced in 250ml Erlenmeyer flask. Fermentation medium consists of 2% dilute sulphuric acid pre-treated saw dust and 1% yeast extract with pH 5. The medium was sterilized at 121oC, 15 Psi pressure for about 15 minutes. After sterilization, the flasks were cooled and inoculated with 2% (v/v) vegetative cells of 24hours old Bacillus subtilis and then it was incubated at 50oC. The agitation speed was maintained at 120 rpm for 24 h. Afterward fermented broth was filtered and centrifuged (Kokusan H- 1500ER) for 10 minutes at 10,000 x g and 4oC for getting clear filtrate as a crude enzyme source. Three readings were taken for every experiment.
Cellulase Assay
CMCase and FPase activity was observed according to the method described earlier [10]. One unit of CMCase / FPase activity is defined as the extent of enzyme needed to liberate 1µ mole of glucose from substrate per millilitre per minute under standard conditions.
Design of Experiment
For optimizing pre-treatment process for maximizing cellulase production, Box-Bhenken design (BBD) was followed in this study. Three factors were optimized i.e. sulphuric acid concentration (A), biomass loading (B) and retention time (C) (Table 1). The design of experiment is appropriate for quadratic response surface and produces second degree polynomial equation. Relationship between coded and actual values were expressed by the following equation (Eq. 1 & 2):
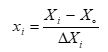
Eq. (1)
Here, xi represents coded value of independent variable.
Xi is encoded value of independent variable.
Xo is encoded value of independent variable at central point
ΔXi is the difference of Xi and Xo.
The response is calculated from the following equation using STATISTICA software (99th edition).
Y = β0+ β1A+ β2B+ β3C+ β11A2+ β22B2+ β33C2+ β12AB+ β13AC+ β23BC Eq. (2)
Y is the response, A, B and C are the independent variables, ß0 is the intercept, ß1,ß2 and ß3 are linear coefficient, ß11, ß22 and ß33 are square coefficients, ß12, ß13 and ß23 are interaction coefficients.
GDxy = l-dxy/dx+dy-dxy
Where GDxy = Genetic distance between two genotypes,
dxy= Total number of common loci (bands) in two genotypes,
dx= Total number of loci (bands) in genotypes,
dy = Total numbers of loci (bands) in genotypes
Results
Enzyme production
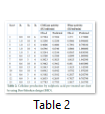
Rate of production of CMSase and FPase was analysed from second order polynomial equations as shown in Eq.3 and 4. Highest CMCase (0.7783 IU/ml/min) and FPase activity (1.3617 IU/ml/min) was observed at optimized condition i.e. 0.8% sulphuric acid concentration, 15g biomass loading and 4h reaction time (Table 2). The predicted and observed values were also found to be very close with values predicted by the model revealing the accuracy of the model (Fig. 1 & 2).
CMCase activity (IU/ml/min) = -3.15 + 4.5 X1 + 0.2592 X2 + 0.321 X3 - 2.81 X12 + 0.00786 X22 - 0.0267 X32 - 0.0261 X1 * X2 + 0.027 X1* X3 - 0.01038 X2* X3 Eq. (3)
FPase activity (IU/ml/min) = – 4.22 + 12.43 X1 + 0.3050 X2 - 0.420 X3 - 7.03 X12 - 0.00630 X22 + 0.0167 X32 - 0.01889 X1* X2 + 0.187 X1* X3 + 0.00101 X2* X3 Eq. (4)
Analysis of variance
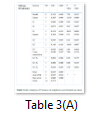
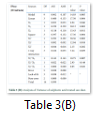
Statistical significance of the data was analysed by ANOVA (Table 3). Proposed model for CMCase and FPase was significant with p-value of 0.017 and 0.005 respectively. Fitness of the model was further analysed by R2 value which was found to be 93.44% and 96.19% for CMCase and FPase respectively for dilute sulphuric acid pre-treatment. While adjusted R2 value for CMCase and FPase was found to be 81.64% and 89.34% respectively which further indicates model precision.
Contour plots
Contour plots for CMCase and FPase produced from dilute sulphuric acid treatment of saw dust is shown in (Fig. 3). These graphs indicated that each parameter had significant effect on cellulase production. The interaction between parameter X1.X2, X1.X3 and X2.X3 had more significant effect as they exhibited circular (dome shape) pattern.
Tables & Figures
Discussion
Lignocellulosic waste is in abundance in our nature like saw dust, bagasse, rice hulls and corn stover. For efficient utilization of lignocellulosic biomass, their complex structure is needed to be disrupted. By pre-treatment, this complex structure disorganizes and further required hydrolysis of cellulose into simple sugars. Hydrolysis is brought about by cellulases which is produced from cellulase producing bacteria and fungi. Different bacterial strains have been explored for producing cellulase enzymes that can withstand high temperature, pH and have high growth rate. Bacillus subtilis (K-18) used in this study is selected owing to high stability, high growth rate, easy availability and good cellulase producing capability.
Cellulase enzyme production was higher in the present study as compared to previous reports. In the present study, dilute sulphuric acid pre-treated saw dust produced 0.773IU/ml/min of CMCase and 1.3617 IU/ml/min FPase by Bacillus subtilis K-18. In one study, pre-treatment of saw dust with 2N NaOH yielded 0.1813IU/ml of cellulase by Aspergillus niger [11]. Another study reported pre-treatment of bagasse, saw dust and corncob with caustic soda produced 0.0743IU/ml cellulase activity from saw dust using Aspergillus flavus. The corncob and bagasse produced 0.0502IU/ml and 0.0573 IU/ml of enzyme activity respectively [12]. The result of another study showed 0.037IU/ml of CMCase produced by Bacillus sp. C1AC5507 from bagasse in submerged fermentation [13]. In submerged fermentation, using Acacia Arabica pod as a substrate maximum cellulase production (0.440 IU/ml/min) was reported by Bacillus cereus [14].
In one study, cellulase production was optimized using 4% NaOH pre-treated saw dust and 0.28IU/g FPase and 5.8IU/g CMCase activity was reported by Aspergillus fumigatus under optimized conditions [15]. Dilute sulphuric acid treatment of eucalyptus leaves yielded CMCase activity of 1.811 IU/ml/min and FPase activity of 2.255 IU/ml/min using Bacillus subtilis K-18 in submerged fermentation [16]. Similarly, peanut shells pre-treated with dilute sulphuric acid gave CMCase units of 1.575 IU/ml/min and FPase units of 2.015 IU/ml/min by the same strain in submerged fermentation [17]. Using same strain and different substrate, the cellulase production become different, for instance, using potato peel as substrate, the CMCase units were 3.50 IU/ml in submerged fermentation [18]. Bacillus aquimaris isolated from the gut of Labeo rhoita has potential of cellulase production using bagasse as substrate in submerged fermentation [19].
The authors declare no conflict of interest.
References
- Yaliwal VS, Adaganti SY, Banapurmath MR, Tewari PG. Renewable and sustainable fuel production from woody biomass. Indian Journal of Chemical Technology, (2015); 22: 61-66.
- Kuhad RC, Gupta R, Singh A. Microbial cellulases and their industrial applications. Enzyme Research, (2011); doi:10.4061/2011/280696.
- Taherzadeh MJ, Karimi K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. International Journal of Molecular Sciences, (2008); 9: 1621–1651.
- Zhang PH, Himmel ME, Mielenz JR. Outlook for cellulase improvement: Screening and selection strategies. Biotechnology Advances, (2006); 24: 452 – 481.
- Kirk OT, Borchert V, Fuglsang CC. Industrial enzyme applications. Current Opinion in Biotechnology, (2002); 13: 345–51.
- Maki M, Leung KT, Qin W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. International Journal of Biological Sciences, (2009); 5:500-516.
- Sadhu S, Maiti TK. Cellulase production by bacteria: a review. British Microbiology Research Journal, (2013); 3: 235–258.
- Tarek AAM, Nagwa AT. Optimization of cellulase and glucosidase induction by sugarbeet pathogen Sclerotium rolfsii. African Journal of Biotechnology, (2007); 6(8): 1048-1054.
- Li W, Du W, Liu DH. Optimization of whole cell-catalyzed methanolysis of soybean oil for biodiesel production using response surface methodology. Journal of Molecular Catalaysis B Enzymatic, (2007); 45:12–127.
- Arooj A, Irfan M, Tabsum F, Shakir HA, Qazi JI. Effect of dilute sulphuric acid pretreatment on cellulase production by Bacillus subtilis K-18 through response surface methodology. Proceedings of the Pakistan Academy of Science B; Life and environmental Science, (2017); 54(1): 11-20.
- Acharya PB, Acharya DK, Modi HA. Optimization for cellulase production by Aspergillus niger using saw dust as substrate. African Journal of Biotechnology, (2008); 7(22): 4147-4152.
- Ojumu TV, Solomon BO, Betiku E, Layokun SK, Amigun B. Cellulase production by Aspergillus flavus Linn Isolate NSPR 101 fermented in sawdust, bagasse and corncob. African Journal of biotechnology, (2003); 2(6):150-152.
- Padilha QM, Carvalho LCT, Dias PVS, Grisi CSL, Honorato da Silva FL, Santos SFM, Araujo DAM. Production and characterization of thermophilic carboxymethyl cellulase synthesized by Bacillus sp. growing in sugarcane bagasse in submerged fermentation. Brazilian Journal of Chemical Engineering, (2015); 32(1).
- Patagundi BI, Shivasharan CT, Kaliwal BB. Isolation and characterization of cellulase producing bacteria from Soil. International Journal of Current Microbiology and Applied Sciences, (2014); 3 (5): 59-69.
- Gilna VV, Khalil KM. Cellulase enzyme activity of Aspergillus fumigatus from mangrove soil on lignocellulosic substrate. Recent Research in Science and Technology, (2011); 3(1): 132-134.
- Iqbal S, Irfan M, Tabassum F, Shakir HA, Qazi JI. Application of Box-Behnken design for optimization of different pre-treatments conditions for cellulase production. Journal of Northeast Agricultural University, (2017); 24(3): 51-59
- Arshad F, Irfan M, Shakir HA, Tabbsum F, Qazi JI. Optimization of dilute sulphuric acid pre-treatments of peanut shells through Box- Bhenken design for cellulase production by Bacillus subtilis K-18. Punjab University Journal of Zoology, (2017); 32 (1): 81-90.
- Irfan M. Mushtaq Q, Tabassum F, Shakir HA, Qazi JI Carboxymethyl Cellulase production optimization from newly isolated thermophilic Bacillus subtilis K-18 for saccharification using response surface methodology. AMB Express, (2017); 7:29.
- Khalid S, Irfan M, Shakir HA, Qazi JI. Endoglucanase producing potential of Bacillus species isolated from the gut of Labeo rohita. Journal of Marine Science & Technology, (2017); 25(5): 581-587.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0/