Full Length Research Article
Assessments of kidney function and morphology of tramadol-diclofenac treated albino rats
Elias Adikwu1,*, Ebinyo C. Nelson2
Adv. life sci., vol. 5, no. 3, pp. 104-112, May 2018
*- Corresponding Author: Elias Adikwu (Email: adikwuelias@gmail.com)
Author' Affiliation
2- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Niger Delta University, Bayelsa State, Nigeria
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Tramadol-diclofenac (TD-DF) could be used in chronic pain management. Concurrent use may present renal complications due to their individual nephrotoxic profile. The present study assessed the kidney function and histology of tramadol-diclofenac treated albino rats.
Methods: Forty two adult albino rats divided into seven groups A-G were used for this study. Rats were orally administered with TD (12 mg/kg/day), DF (6 mg/kg/day), and TD-DF for 14 days including two recovery groups. Rats were weighed and sacrificed at the termination of drug treatment. Serum was extracted from blood and evaluated for creatinine (Cr), urea (U), uric acid (UA), total protein (TP), albumin (Ab) and serum electrolytes (K+, Na+, Cl-, and HCO3-). Kidneys were excised weighed and evaluated for alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), superoxide dismutase (SOD), catalase (CAT) glutathione (GSH), glutathione peroxidase (GPX), malondialdehyde (MDA) levels and histological damage.
Results: The body weight, absolute and relative kidney weights and serum electrolytes were not significantly (p> 0.05) altered in the TD-DF treated rats in comparison to control. However, the levels of Cr, U, UA, AST, ALT, ALP and MDA were significantly (p<0.05) increased whereas Ab, TP, SOD, GSH, GPX and CAT were significantly (p<0.05) decreased in the TD-DF treated rats in comparison to treatments with individual doses of TD and DF. Varying degrees of histological damage were observed in the kidneys of TD-DF treated rats. However, nephrotoxic effects due to treatment with TD-DF were reversed in the recovery groups.
Conclusion: The use of tramadol-diclofenac could be associated with reversible nephrotoxicity; therefore renal function assessment is advised before tramadol-diclofenac use.
Keywords: Tramadol, diclofenac, co-treatment, kidney, toxicity, rats
In clinical practice, drugs are concurrently used in the treatment of severe and chronic pains that are irresponsive to monotherapy. In spite of the benefits expected from concurrent use of drugs there could be possible interactions that may lead to undesired or unexpected outcomes such as toxicities. In some instances the undesired or unexpected outcomes may outweigh the desired therapeutic benefits [1,2]. In the clinical assessment of possible toxicities that could arise as a product of drug interactions, some primary factors are taken into consideration. These factors include the patient age, duration of therapy, chemical nature of drugs, the number of drugs used and the status of the liver and kidney. The kidney is known to be one of the primary organs vulnerable to drug interactions which could impair its structure and functions. Kidney serves several, essential regulatory and excretory functions. These functions include maintenance of acid–base balance, electrolytes regulation, disposal of nitrogenous wastes, removal of drugs and their biotransformed products [3]. Also, it is responsible for the production of essential biochemical substances such as rennin, erythropoietin and calcitriol and the reabsorption of glucose, water, and amino acids [4]. Kidney as the primary excretory organ for drugs, chemicals and biotransformed products is vulnerable to toxicity. This calls for its constant clinical assessment during therapy with drugs to safeguard it from toxicity [5].
Diclofenac (DF) has proven to be effective in the treatment of pain and is one of the commonly prescribed non steroidal anti-inflammatory drugs (NSAID). In addition to its analgesic effect, it has anti-inflammatory and antipyretic properties which make it suitable for the treatment of both peripheral and central pains. Studies have shown that its clinical benefit is due to the prevention of prostaglandin synthesis by inhibiting the activities of cyclooxygenase-1 and cyclooxygenase-2 [6]. However, the inhibition of renal prostaglandin synthesis by DF has been associated with nephrotoxicity. Reported nephrotoxicity with the use of DF could be characterized by alterations in renal biomarkers and kidney architecture [7,8]. Also, animal studies reported dose-dependent effects on kidney-linked stereological parameters such as renal damage, oxidative stress, plasma coagulation parameters and adenosine deaminase activity [9-11].
Tramadol (TD) is an opioid analgesic that acts centrally; it has structural similarity with codeine and morphine. It is commonly and effectively used in the treatment of acute and chronic pains. Its beneficial effect is attributed to agonist action at the mu opioid receptor, and the inhibitory effect on norepinephrine and serotonin reuptake [12]. It has a good oral absorption; it is rapidly and almost completely absorbed and well distributed. TD and biotransformed products are excreted by the kidney via urine consequently; this makes the kidney a primary target organ of TD toxicity especially in cases of misuse and over dose. Some features of kidney damage associated with TD are cellular degeneration characterized by vacuolization, and tubular necrosis. Also, impaired levels of serum renal markers characterized by depletion of kidney antioxidant defence were features observed in animal studies [13].
Furthermore, because of failure of monotherapy, TD and DF could be used concurrently in the treatment of chronic pain. Concurrent use could be beneficial in ameliorating or abolishing both peripheral and central pains. Also, length of therapy could be reduced; analgesia could be produced at lower and more tolerable doses of the constituent drugs. Concurrent use could increase the ability of individual drugs to decrease pain, with increase tolerability and enhanced recovery time [14]. However, both drugs are known to be excreted by the kidney and are individually associated with nephrotoxicity. Therefore, concurrent use may precipitate drug interaction that may be characterized by nephrotoxic consequence which may lead to structural and functional changes in the kidney. However, in the absence of literature on the effect of diclofenac-tramadol on the kidney, this study assessed the renal effect of tramadol-diclofenac administration in albino rats.
Animals
The rats used for this study were obtained from the animal house of the Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State. The rats were housed as six per cage and had free access to food and water ad libitum.
Drugs
Tramadol capsule used for this study was manufactured by Osaka Pharmaceutical India while diclofenac tablet was manufactured by Exus Pharmaceutical Nigeria. All other chemicals used are of analytical standard.
Experimental protocol
Forty two adult albino rats used for this study were divided into seven groups A-F with six rats in each group. Rats in groups A and B were treated with water and normal saline respectively as placebo and solvent control. Rats in groups C-E were treated with TD (12mg/kg/day), DF (6mg/kg/day) and TD-DF for 14 days respectively. Rats in groups G and F (recovery groups) were treated with TD-DF orally for 14 days and allowed for 7 and 14 days wash-out periods respectively. The doses of tramadol and diclofenac used for this study represent 2 times maximum daily clinical doses.
Collection of sample
At the end of drug administration, rats were sacrificed using inhalational diethyl ether as anaesthesia. Blood samples were collected, centrifuged at 1500 rpm for 20 minutes and serum extracted and evaluated for renal function parameters. The kidneys were collected via dissection and washed in an ice cold 1.15% KCl solution. The kidneys were homogenized with 0.1M phosphate buffer (pH 7.2) and centrifuged at 1500 rpm for 20 minutes. The supernatant was decanted and evaluated for oxidative stress indices and biochemical parameters.
Evaluation of biochemical parameters
Jaffe’s method was used for the evaluation of creatinine, diacetylmonoxime method was used for the evaluation of urea while uric acid was evaluated using phosphotungstic acid method [15]. Albumin was determined using end point method while total protein level was assayed using Biuret method [16]. The method of Reitman and Frankel (1957) [17] was used for the evaluation of aspartate transaminase and alanine transaminase. Alkaline phosphatase was assayed using phenolphthalein method [18]. Kidney Superoxide dismutase activity was determined according to Sun and Zigma [19] while catalase activity was determined according to Sinha, et al. [20]. Reduced glutathione (GSH) was estimated as reported by Sedlak and Lindsay [21] while glutathione peroxidase was analyzed as reported by Rotruck et al. [22]. Malondialdehyde (MDA)was determined as described by Buege and Aust [23]. Flame photometric methods were used for the evaluation of potassium and sodium while titrimetric method was used for chloride analysis. Bicarbonate level was evaluated using standard laboratory test kits.
Histological examination of the kidney
Kidney tissues were collected from different groups and were fixed in 10% buffered formalin solution. The tissues were processed, embedded in paraffin and sections of 5µm thickness were obtained. The sections were stained with hematoxylin and eosin and examined using a light microscope.
Statistical analysis
Data are represented as means ± SD. The differences were compared for statistical significance by ANOVA and Tukey’s post-hoc test. Difference was considered significant at p < 0.05.
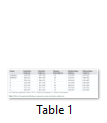
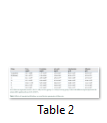
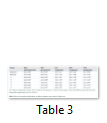
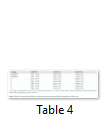
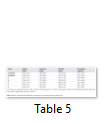
Body weight, absolute and relative kidney weights and serum electrolytes (K+, Na+, Cl- and HCO3-) were not significantly (p>0.05) altered in TD-DF treated rats when compared to control (Table 1 and 3). On the other hand, serum Cr, U and UA were significantly (p<0.05) increased in TD-DF treated rats when compared to treatments with individual doses of TD and DF (Table 3). Furthermore, serum levels of TP and Ab were decrease in TD-DF treated rats. The observed decreases in serum TP and Ab levels were significantly (p<0.05) different when compared to treatments with individual doses of TD and DF (Table 3). Furthermore, in the TD-DF treated rats, MDA levels were increased while SOD, CAT and GSH levels were decreased. Comparatively, effects on these parameters differ significantly (p<0.05) in comparison to effects produced by their individual doses (Table 4). The kidney levels of AST, ALT and ALP were increased by 120.3%, 106.1% and 125.7% respectively in TD-treated rats.
These parameters were increased by 111.1%, 100.5% and 131.1 % in DF-treated rats while 278.5%, 304.7% and 350.8 % increases were obtained in TD-DF treated rats respectively.
The increases observed in AST, ALT and AST in rats treated with TD-DF differ significantly (p<0.05) when compared to individual doses of TD and DF (Table 5). However, the altered levels of evaluated parameters in TD-DF treated rats were significantly (p<0.05) restored in the recovery groups allowed for a wash-out period of 7 and 14 days respectively when compared to TD-DF treated rats. In addition, the kidney of the control rat administered with water showed normal histology (Figure A). The kidneys of rats treated with TD showed dilated tubules and necrosis of tubular epithelium, also, the kidneys of DF treated rats showed dilated tubules and necrosis of tubular epithelium. Furthermore, the kidneys of TD-DF treated rats showed dilated tubules, necrosis of tubular epithelium and eosinophylic materials in the tubular lumen. The kidneys of the recovery group allowed for a wash-out period of 7 days showed tubular necrosis while the recovery group allowed for a wash-out period of 14 days showed normal kidney histology (Figure B-F).
Body and organ weights are key indices for the toxicological assessments of drugs because they could be modified with the advent of drug-induced toxicity [24]. In the present study, body weight, absolute and relative kidney weights were not altered in TD-DF treated rats. Serum biochemical markers are integral component and tools used for the assessments of the toxicological profile of xenobiotics. Serum electrolytes are essential renal biochemical markers. They are associated with vital functions which include the maintenance of acid–base balance, osmotic pressure, movement and regulation of fluids and muscular activity. Renal toxicity associated with drugs can be characterized by serum electrolyte fluctuations which could be detrimental to health [25,26]. This study observed normal levels of serum electrolytes in TD-DF treated rats. In clinical practice, serum creatinine level is widely used to estimate glomerular filtration rate and it serves as an index for renal function assessment [27]. Urea which is a nitrogenous waste is produce from the metabolism of protein and amino acid and is primarily eliminated through urinary excretion. It is an essential clinical parameter for the assessment of the nephrotoxic profile of xenobiotics. Uric acid is the product of the oxidation of purine which it is excreted through the urine [28]. The assessments of creatinine, urea, and uric acid levels can be correlated with the functional status of the kidney [29,30]. The observations in the current study showed increased serum levels of creatinine, urea, and uric acid in TD-DF treated rats. This finding is a sign of renal toxicity which might have instigated decrease in glomerular filtration rate leading to the build-up of creatinine, urea and uric acid in the blood. Clinical diagnosis have shown that decreases in serum concentrations of protein and albumin characterized by significant increases in the urinary excretion of protein and albumin are indicators of renal dysfunction [30].. The observations in this study showed decreases in the serum concentration of total protein and albumin in TD-DF treated rats which indicate kidney damage. Furthermore, kidney levels of AST, ALT and ALP were increased in TD-DF treated rats in comparison to treatment with individual doses TD and DF. Superoxide dismutase(SOD) catalytically convert super oxide anion to oxygen and hydrogen peroxide [31] while catalase (CAT) inhibits cell damage by degrading hydrogen peroxide to water and oxygen [32]. Glutathione (GSH) contains thiol group stored as cysteine residue, it acts by inhibiting activities of reactive oxygen/nitrogen species and electrophiles and as a cofactor for enzymes [33,34]. Glutathione peroxidase (GPx) is a selenoenzyme that facilitates the activity of glutathione through the reduction of harmful peroxides. Also, it actively protects lipid membranes and other cellular components from harmful effect of oxygen/nitrogen species [35]. Due to the modulatory effects of SOD, CAT, GSH and GPX on oxidative processes, their concentrations are often correlated with oxidative stress [36,37]. The kidney levels of SOD, CAT, GSH and GPX were decreased in the TD-DF treated rats. The observation could be as a result of the induction of oxidative stress by TD-DF through the production of oxidative radicals in the kidneys of treated rats. Lipid peroxidation is a free radical reaction, stimulated by the interaction of oxidative radicals with unsaturated lipids present in bio-membranes. This will result in the production of lipid hydro peroxide, lipid peroxide radicals, and products such as malondialdehyde (MDA). Studies use MDA as a basic biological tool for the assessment of lipid peroxidation and its concentration is usually high when oxidative stress is established [38]. The current study observed elevated level of MDA in TD-DF treated rats. This showed that nephrotoxicity due to TD-DF could be associated with lipid peroxidation. Conventional histological stains such as haematoxylin and eosin are commonly used for the preparation of tissues for histological evaluation [39]. Histological assessment of the haematoxylin and eosin stained sections of the kidneys of rats treated with TD-DF showed varying degrees of architectural distortions. This observation could be attributed to the induction of oxidative stress and lipid peroxidation through oxidative radical generation by TD-DF. Empirical evidence has shown that the production of oxidative radicals in biological systems primarily hydroxyl radicals, peroxy radicals and superoxide anion can damage bio-membranes and biomolecules like lipids, amino acids and nucleic acids. Also, free radicals can denature proteins in kidneys leading to aggregation, loss of function, cross-linking, and destruction of connective tissues [40]. However, the most destructive effect of oxidative radicals is the induction of lipid peroxidation through the breakdown of poly-unsaturated fatty acid component of cells [41]. In this study, the altered levels of evaluated biochemical parameters correlate with histopathological damage observed in the kidneys of TD-DF treated rats. It is of interest to note that renal toxicity observed in TD-DF treated rats were reversed in the recovery groups. However, recovery was more prominent in the 14 days recovery group. These findings showed that renal toxicity associated with the use of TD-DF could reverse with time.
Furthermore, in this study, observations in the TD-treated rats are consistent with the work of Alkhateeb et al. who reported elevated levels of creatinine and urea and tubular necrosis in the kidneys of rats treated with 30mg/kg of TD for 30 days [42]. Abdel-Zaher et al. reported altered levels of oxidative stress biomarkers in TD-treated mice [43]. Renal insufficiency due to the decreased glomerular filtration rate characterized by increase oxidative radical production was reported as possible cause of TD-induced nephrotoxicity [44]. Also, observations in DF-treated rats are consistent with the work of Yasmeen et al. who reported increases in creatinine, urea and uric acid and tubular necrosis in rats administered with 2mg/kg/day of DF for 14 days [45]. Also, Basavraj et al observed increases in serum creatinine and urea with decreases in total protein and albumin in mice- treated orally with 9.5 mg/kg/day of DF for 28 days [46]. In addition, El-Maddawy et al. reported altered levels of oxidative stress indices in the kidneys of rats administered with 13.5 mg / kg/day of DF intramuscularly for 14 day [47].
Studies have attributed the nephrotoxic effect of DF to the inhibition of renal prostaglandin synthesis, which influences cortical blood flow, glomerular filtration rate and salt and water excretion [48]. The use of tramadol-diclofenac could be associated with reversible nephrotoxicity. The present study recommends renal function assessment before use.
Acknowledgement
The authors appreciate the technical assistance offered by the Joeman Adaeze, Boutiti Promise and Cosmos Obi of the Department of Pharmacology and Toxicology, Niger Delta University Amassoma.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Leone R, Magro L, Moretti U, Cutroneo P, Moschini M, et al. Identifying adverse drug reactions associated with drug-drug interactions: data mining of a spontaneous reporting database in Italy. Drug Safety, (2010); 33(8): 667-675.
- Pirmohamed M. Drug-drug interactions and adverse drug reactions: separating the wheat from the chaff. Wiener klinische Wochenschrift, (2010); 122(3-4): 62-64.
- Aronson JK. Drugs and renal insufficiency. Medicine, (2007); 35(7): 396-398.
- Raghavendra M, Vidya M. Functions of kidney & artificial kidneys. International Journal of Innovative Research, (2013); 1(11): 1-5.
- Doogue MP, Polasek TM. Drug dosing in renal disease. The Clinical Biochemist Reviews, (2011); 32(2): 69.
- Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Current medical research and opinion, (2010); 26(7): 1715-1731.
- Palmer BF. Renal complications associated with use of nonsteroidal anti-inflammatory agents. Journal of investigative medicine: the official publication of the American Federation for Clinical Research, (1995); 43(6): 516-533.
- O'connor N, Dargan PI, Jones AL. Hepatocellular damage from non-steroidal anti-inflammatory drugs. Qjm, (2003); 96(11): 787-791.
- Pazvant G, Sahin B, Kahvecioglu KO, Gunes H, Ince NG, et al. The volume fraction method for the evaluation of kidney: A stereological study. Ankara Univ Vet Fak Derg, (2009); 56233-239.
- Altan F, Elmas M, Er A, Uney K, Cetin G, et al. Effects of drugs on kinetic values of cytokines, adenosine deaminase and 13, 14-dihydro-15-keto-prostaglandin F2α in endotoxemia: a different approach. Eurasian Journal of Veterinary Sciences, (2010); 26(1): 15-19.
- Musu M, Finco G, Antonucci R, Polati E, Sanna D, et al. Acute nephrotoxicity of NSAID from the foetus to the adult. European review for medical and pharmacological sciences, (2011); 15(12): 1461-1472.
- Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clinical Pharmacokinetics, (2004); 43(13): 879-923.
- Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, et al. Liver and kidney toxicity in chronic use of opioids: an experimental long term treatment model. Journal of Biosciences, (2005); 30(2): 245-252.
- Raffa R. Pharmacology of oral combination analgesics: rational therapy for pain. Journal of Clinical Pharmacy and Therapeutics, (2001); 26(4): 257-264.
- Patricia OO, Christiana BA, Raphael OJ. Evaluation of changes in renal functions of pregnant women attending ante-natal clinic in Vom Plateau State, North-Central Nigeria. Archive of Applied Science Research, (2013); 5(4): 111-116.
- Zainulabdeen JA, Alak SA. Effects of smoking on protein level and alpha amylase activity in sera of Iragi Narghile smokers Al. Mustansiriyah Journal of Sience (2014); 25(3): 35-40.
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American journal of clinical pathology, (1957); 28(1): 56-63.
- Babson AL, Greeley SJ, Coleman CM, Phillips GE. Phenolphthalein monophosphate as a substrate for serum alkaline phosphatase. Clinical Chemistry, (1966); 12(8): 482-490.
- Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Analytical biochemistry, (1978); 90(1): 81-89.
- Sinha AK. Colorimetric assay of catalase. Analytical biochemistry, (1972); 47(2): 389-394.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical Biochemistry, (1968); 25192-205.
- Rotruck JT, Pope AL, Ganther HE, Swanson A, Hafeman DG, et al. Selenium: biochemical role as a component of glutathione peroxidase. Science, (1973); 179(4073): 588-590.
- Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods in enzymology: Elsevier. pp. 302-310.
- Bailey SA, Zidell RH, Perry RW. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicologic Pathology, (2004); 32(4): 448-466.
- Hussain F, Maan MA, Sheikh MA, Nawaz H, Jamil A. Trace elements status in type 2 diabetes. Bangladesh Journal of Medical Science, (2009); 8(3): 52.
- Yunos NaM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Critical Care, (2010); 14(4): 226.
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clinical Chemistry, (1992); 38(10): 1933-1953.
- Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: a danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutionary considerations; 2011. Elsevier. pp. 394-399.
- Zuo Y, Wang C, Zhou J, Sachdeva A, Ruelos VC. Simultaneous determination of creatinine and uric acid in human urine by high-performance liquid chromatography. Analytical Sciences, (2008); 24(12): 1589-1592.
- Garg S, Gupta AK, Rohtgi A, Sharma S. Evaluation of Random Urine Sample Protein-Cretinine Ratio as an Index of Quantitative Proteinuria. JK Science, 6(3): 134-137.
- Fridovich I. Superoxide radical and superoxide dismutases. Annual Review of Biochemistry, (1995); 64(1): 97-112.
- Alfonso-Prieto M, Biarnés X, Vidossich P, Rovira C. The molecular mechanism of the catalase reaction. Journal of the American Chemical Society, (2009); 131(33): 11751-11761.
- Sies H. Glutathione and its role in cellular functions. Free Radical Biology and Medicine, (1999); 27(9-10): 916-921.
- Cooper AJ, Pinto JT, Callery PS. Reversible and irreversible protein glutathionylation: biological and clinical aspects. Expert opinion on drug metabolism & toxicology, (2011); 7(7): 891-910.
- Mugesh G, Panda A, Singh HB, Punekar NS, Butcher RJ. Glutathione peroxidase-like antioxidant activity of diaryl diselenides: a mechanistic study. Journal of the American Chemical Society, (2001); 123(5): 839-850.
- Huang H-S, Ma M-C, Chen J, Chen C-f. Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. The Journal of urology, (2002); 167(6): 2584-2593.
- Ozbek E. Induction of oxidative stress in kidney. International journal of nephrology, (2012); 2012(465897).
- Gaweł S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci lekarskie (Warsaw, Poland: 1960), (2004); 57(9-10): 453-455.
- Moreso F, Lopez M, Vallejos A, Giordani C, Riera L, et al. Serial protocol biopsies to quantify the progression of chronic transplant nephropathy in stable renal allografts. American Journal of Transplantation, (2001); 1(1): 82-88.
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiological Reviews, (1979); 59(3): 527-605.
- Repetto MG, Ferrarotti NF, Boveris A. The involvement of transition metal ions on iron-dependent lipid peroxidation. Archives of Toxicology, (2010); 84(4): 255-262.
- El-Khatib AS, Moustafa AM, Abdel-Aziz A-AH, Al-Shabanah OA, El-Kashef HA. Effects of aminoguanidine and desferrioxamine on some vascular and biochemical changes associated with streptozotocin-induced hyperglycaemia in rats. Pharmacological research, (2001); 43(3): 233-240.
- Abdel-Zaher AO, Abdel-Rahman MS, ELwasei FM. Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: role of nitric oxide and oxidative stress. Neurotoxicology, (2011); 32(6): 725-733.
- Awadalla EA, Salah-Eldin A-E. Histopathological and molecular studies on tramadol mediated hepato-renal toxicity in rats. IOSR Journal of Pharmacy and Biological Sciences, (2015); 10(6): 90-102.
- Yasmeen T, Qureshi GS, Perveen S. Adverse effects of diclofenac sodium on renal parenchyma of adult albino rats. Journal of Pakistan Medical Association, (2007); 57(7): 349-351.
- Thanagari BS, Fefar DT, Prajapati KS, Jivani B, Thakor KB, et al. Haemato-biochemical alterations induced by diclofenac sodium toxicity in Swiss albino mice. Vet World, (2012); 5417-419.
- El-Maddawy ZK, El-Ashmawy IM. Hepato-renal and hematological effects of diclofenac sodium in rats. Global Journal of Pharmacology, (2013); 7(2): 123-132.
- Murray M, Brater DC. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annual Review of Pharmacology and Toxicology, (1993); 33(1): 435-465.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0