Full Length Research Article
Antioxidant Potential of Lactarius deliciosus and Pleurotus ostreatus from Amanos Mountains
Adnan Bozdogan*, Zeynep Ulukanlı, Fuat Bozok, Tülin Eker, Hasan Hüseyin Doğan, Saadet Büyükalaca
Adv. life sci., vol. 5, no. 3, pp. 113-120, May 2018
*- Corresponding Author: Adnan Bozdogan (Email: bozdogan@osmaniye.edu.tr)
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Edible mushrooms are considered as the significant source of minerals and vitamins. Lactarius deliciosus and Pleurotus ostreatus are the two known edible mushroom species because of their distinctive taste as well as nutritional properties.
Methods: L. deliciosus and P. ostreatus were collected from the native flora of Amanos Mountains in Turkey. The methanol (MeOH) extract of the L. deliciosus and P. ostreatus was obtained to assess antioxidant potential, Total phenolic, flavonoid, β-carotene and lycopene content.
Results: Total phenolic (mg/kg), flavonoid (mg/kg) and β-carotene (mg/100 ml) contents of the MeOH extracts of L. deliciosus and P. ostreatus were 34.55, 6.03, 1.15 and 15.66, 0.16, 0.02, respectively. Lycopene was only detected in L. deliciosus with a content of 0.25 (mg/100 ml). At 5 mg/ml, DPPH (%), RP (Abs.), H2O2 (%), NO (%) and FRAP (mmol Fe2+/L) activities of L. deliciosus and P. ostreatus were 45.33, 0,790, 88.30, 55.51, 0.57 and 17.42, 0,530, 73.77, 33.64, 0.28, respectively.
Conclusion: Pearson correlation among the antioxidant results was found to be well correlated (r ≥0.90). Antioxidant results were highly correlated (r ≥0.93) with total phenolic compounds and lycopene. As of date, there has no previous work not only the NO but also H2O2 radical scavenging capabilities of L. deliciosus and P. ostreatus.
Keywords: Lactarius, Pleurotus, antioxidant, scavenging, Amanos
The combination of substances with oxygen causes losing electrons and atoms and yield energy, which also forms a significant source of fuels for the fundamental process in the livings to sustain their life [1]. Some illness e.g. cancer, diabetes, rheumatism, cirrhosis, arteriosclerosis and etc. could be resulted from a variety of free radicals and reactive oxygen species generated in the cell nature [1-4]. In a daily life, edible food has the significant sources of natural constituents might be helpful for living things to reduce damages derived from oxidation [5-7]. Edible mushrooms are considered as the significant source of minerals and vitamins [8-10]. Today, mushroom consumption is steadily increasing due to developments in cultivation techniques [11]. Antioxidant activities of these group of organisms were previously investigated by many authors [11-19]. Turkey has a great potential in terms of natural edible mushrooms and is becoming a major exporter in the world [20]. Amanos Mountains, also known as Nur or Gavur, are located in South of Turkey. Amanos Mountains have been surrounded by Osmaniye (Duzici) and Kahramanmaras in North, Gaziantep (Islahiye) in East, Hatay (Samandag) in South. The highest point of Amanos Mountains is Boztepe (2240 m). Amanos, is the place important for bio diversification, which include 1580 plant species and 251 of those are endemics.
In the present work, phenol, flavonoid, β-caroten and lycopene contents, antioxidant activities including DPPH, RP, NO, FRAP and H2O2 potential of the MeOH extract prepared from L. deliciosus and P. ostreatus collected from the native flora of Amanos Mountains were analyzed. Antioxidant potential of L. deliciosus and P. ostreatus, could be the first investigation in the Amanos region.
Fungal Samples and Extraction
Lactarius deliciosus and Pleurotus ostreatus were collected from Cebel (37o01’17” N, 36o22’10” E, 988 m) and Türkü (37o02’01” N, 36o28’18” E, 1189 m) regions of Amanos Mountains (Yarpuz-Osmaniye, Turkey, on 15 November 2014). After collection, mushroom samples were dried in a dehydrator for 48 h and then powdered. Dried samples were pulverized in a blender (Waring Blender, HGB2WTS3). Extraction step was carried out using the powder (50 g) in 400ml of the MeOH solvent at 65 °C for 72 h and this followed by removing the solvent in a rotary evaporator at 40 ºC and then preserved at refrigerated state until use. The yield (%, w/w) was 10.62 in Lactarius deliciosus and 6.30 in Pleurotus ostreatus, respectively.
Total Phenolics (TP)
Folin-Ciocalteu (FC) technique was used to determine the phenolic compounds of the samples [13]. Distilled water and FC reagent (45: 1 ml, 2 N) were transferred into flask including the test extract (1 ml) from the stock solution (2 mg/ml), respectively. After 5 minutes, Na2CO3 solution (3.0 ml, 2.0%) was pipetted and incubated (2 h) in the dark. At the wavelength of 760 nm in a spectrophotometer (UV 1800 Shimadzu). As a standard, gallic acid ranged from 0 to 100 mg/l was used for preparing the slope.
Total Flavonoids (TF)
The modification of Dowd technique used by Gursoy et al. was employed [13]. As described in total phenolic contents, aliquot from the stock solution (1 ml): equal amount of aluminium trichloride solution (2%, AlCl3) was kept for 10 min. Blank had only extract solution and MeOH (1:1, ml). Following the incubation, reaction was assessed versus MeOH blank, at the wavelength of 415 nm. Quercetin at varies concentrations were prepared to compare the results (mg QE/kg).
β-Carotene and Lycopene Content
The method and the formula used by Barros et al. were employed for these chemical parameters in this assay [21]. 0.1 g of the extract: 10 ml of acetone: hexane solution (4:6) was thoroughly shaken for 60 sec. The solution was filtered and then filtrate was read in comparison to blank (acetone: hexane solution), at four different wavelengths (453, 505, 645 and 663 nm). The results were calculated on the basis of the following equations:

Antioxidant Assays
In all assays, BHT was tested as the standard to check the potential of the tested substances.
Reducing Power Assay
Oyaizu method was employed for testing the reducing power activity of the extract solution [22]. In this method, equal volumes (2.5) of the extract solution, buffer (0.2 M sodium phosphate, pH 6.6) and tripotassium hexacyanoferrate (III) (1%) in a tube was vortexed and kept for incubation (50 oC/ 20 min). After addition of the same amount of trichloroethanoic acid (10%, w/v), tube was spinned (1000 rpm/8 min). Supernatant and distilled water in equal volume (5:5, ml) and iron chloride (1 ml, 0.1%) was added to a tube and then vortexed. To measure the abs. value of the reaction was employed at 700 nm.
DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
Aliquot from each concentration of the test substance and DPPH solution in MeOH (60 mM) (0.1:3.9 ml) in a test tube was vortexed and then maintained without light exposure for 1 h. Measurement of the abs. in the solution was performed at 515 nm in comparison to blank (MeOH). % inhibition of the DPPH was assessed using the equation as below: Asample is the abs. value of tested substance and Acontrol is the abs. value of the DPPH [23].
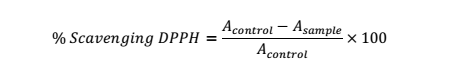
Ferric Reducing Antioxidant Power (FRAP)
A fresh reagent before each testing was made and consisted of 1 ml of TPTZ solution (10 in 40 mmol/l HCl), 1 ml of FeCl3 (20 mmol/l) and 10 ml of acetate buffer (0.1 mol/l, pH 3.6) at 37 °C/10 min [24]. Test substance in MeOH and the reagent (0.3: 2, ml) was subsequently added into a flask and then adding distilled water up to 10 ml of final volume. After incubation period (10 min), blue colour appeared in the solution. The measurement of the Abs. value of the solution versus blank (reagent solution only) was done at 593 nm. A curve was made with different concentrations of FeSO4·7H2O at ranging from 0.2 to 2 mmol/l.
Nitric Oxide (NO) Scavenging Activity
Nitric oxide scavenging activity was carried out with the Griess Illosvoy reaction [25]. Test solution: Phosphate buffer (0.1M, pH 7.0): sodium nitroprusside solution (10mM) (0.5:0.5:2, ml) was subsequently added into test tube and then vortexed. This was then followed by incubating the solution at room temperature for 2.5 h. Equal volumes of the reacting solution and Griess reagent (1.25:1.25 ml) was maintained for incubation (30 min). Abs. value of the reacting solution versus distilled water as the control was done at 548 nm.
NO (%) was based on the following equation:
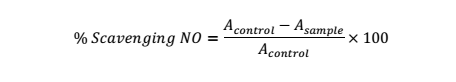
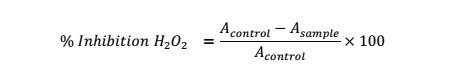
H2O2 Scavenging Activity
In this test, varying concentrations of the test substance (0.0156 to 0.125 mg/ml) were assayed [26]. Test solution: H2O2 solution (40 mmol/l in phosphate buffer): phosphate buffer (pH 7.4) at 1:0.6:3.4 ml was added to test tube. Abs. of the reacting solution versus blank including extract solution plus phosphate buffer (1:4, ml) was checked spectrophotometrically at 230 nm. The control consisted of phosphate buffer: H2O2 solution (3.4:0.6, ml). The equation was used for % H2O2 inhibition.
Statistical Analysis
Data were analysed by Pearson correlation method using SPSS statistics software version 18.0 (SPSS Inc., Chicago, IL, USA).
In the present study, the results of total phenolic (mg/kg), flavonoid (mg/kg) and β-carotene (mg/100 ml) contents of L. deliciosus and P. ostreatus in Amanos Mountains were determined as 34.55, 6.03, 1.15, and 15.66, 0.16, 0.02, respectively (Table 1). Unlike P. ostreatus, L. deliciosus had lycopene (mg/100 ml) content of 0.25. As a result, it was found that total phenolic, flavonoid, β-carotene and lycopene contents of L. deliciosus were higher than that of P. ostreatus.
Antioxidant Activities of Mushrooms
Results of the antioxidant activity of the MeOH extract obtained from each mushroom are shown in Figure 1.
DPPH Scavenging Activity (DSA)
DPPH activities of MeOH extracts of L. deliciosus and P. ostreatus in Amanos Mountains were tested at 1.25, 2.5 and 5 mg/ml. At 5.0 mg/ml, L. deliciosus and P. ostreatus revealed highest DPPH activity with 45.33% and 17.42%, respectively. DPPH activities of BHT had 96% at the same concentration.
Reducing Power (RP)
RP of L. deliciosus and P. ostreatus had concentration-dependent activities. RP (Abs.) of MeOH extracts at 1.25, 2.5 and 5 mg/ml were 0.28, 0.54, 0.79 in L. deliciosus and 0.15, 0.27, 0.54 in P. ostreatus, respectively.
Nitric Oxide Scavenging Activity (NOSA)
In this study, NOSA (%) of L. deliciosus and P. ostreatus were 55 and 33 at 5.0 mg/ml, respectively, whereas NOSA (%) of BHT was 51 at 5.0 mg/ml. It appeared that NOSA of L. deliciosus was higher activity than those of P. ostreatus and BHT.
Hydrogen Peroxide (H2O2) Scavenging Activity (HPSA)
HPSA (%) of L. deliciosus, P. ostreatus and BHT were 88.30, 73.77 and 93.76 at 5.0 mg/ml, respectively.
Ferric-Reducing Antioxidant Power (FRAP)
FRAP (mmol Fe+2/L) activities of L. deliciosus and P. ostreatus in Amanos Mountains revealed concentration dependent activities. As per results, FRAP activities at 1.25, 2.5 and 5.0 mg/ml were 0.10, 0.26, 0.57 in L. deliciosus and 0.03, 0.11, 0.28 in P. ostreatus, respectively.
The antioxidant activities as well as antioxidant content of L. deliciosus and P. ostreatus are given in Table 1. A good correlation (r≥0.90) was observed among the antioxidant results when using different methods. In the present study, antioxidant results were also well-correlated (r≥0.93) with total phenolic compounds and lycopene. In addition, β-carotene content was correlated with DPPH, RP, NO and FRAP. Flavonoid contents were also correlated with DPPH, RP and NO.
Tables & Figures
Phenolic compounds are important compounds that contribute to coloration and organoleptic properties of plants and fruits [27]. Flavonoids are low molecular weight polyphenolic compounds [28]. Phenolic compounds inhibit the oxidation in vitro LDL (low-density lipoprotein) and play important roles on the elimination effects of hypertension, absorption of glucose and the growth of tumors by neutralizing the free radicals [29-33]. β-carotene is a pigment and soluble in oil, which is a precursor of vitamin A in mammals [34]. The lycopene is a well known compound as the isomer of β-carotene, which is synthesized to absorb light during photosynthesis by plants and microorganisms [35]. β-carotene and lycopene function as the antioxidants by scavenging free radicals [36].
Total phenolic and flavonoid contents were expressed as equivalent to gallic acid and quercetin (mg/kg). In previous studies, total phenolic and flavonoid contents of various extracts of L. deliciosus and P. ostreatus varied in different habitats. It was reported that total phenolic content (mg/g) of L. deliciosus was 1.5 in Spain [11], 51.27 in Bolu region of Turkey [37] and 17.25, 24.0 in Bragança and Trás-os-Montes regions of Portugal [38,39]. In addition, flavonoid content (mg/g) of L. deliciosus was 3.0 in Spain [11]. Total phenolic and flavonoid contents (mg/g) in the MeOH extract of P. ostreatus were 1.6 and 1.0 in Spain [11], 1.44 and 0.37 in Poland, respectively [40]. In Korea, total phenolic contents (mg/g) of yellow, pink and dark grey strain of P. ostreatus were 39.3, 30.1, 21.2 and flavonoid contents were 1.96, 1.21, and 2.16 respectively [41]. Total phenolic contents (mg/g) of P. ostreatus MeOH extracts were 1.32 in India [42], 12.1 in Blacksea region of Turkey [7], and 42.47 in Thailand [43].
There have been limited reports on the presence of β-carotene and lycopene contents of mushroom species. In the first report, β-carotene and lycopene contents (mg/g) in MeOH extract of P. ostreatus were determined as 0.317, 0.195, respectively [40]. In the second report, β-carotene was not determined in P. ostreatus collected from Black Sea region of Turkey [7].
DPPH is one of the mostly accepted methods for assessing the antioxidant activity [44]. DPPH radical has an advantage because of not affected by side reactions. Antioxidant agents neutralize the DPPH radical by donating an electron or the hydrogen atom and radical scavenging capacity of DPPH could be determined by reading absorbance at 517 nm by spectrophotometry with a colour change from purple to yellow [45].
The present results showed some differences than that of Akata et al. [16] who reported that DPPH activity of L. deliciosus at 2.56 mg/ml was 92.02%, and P. ostreatus at 2.72 mg/ml was 96.16%. DPPH activity of MeOH extract at 50 mg/ml of L. deliciosus was 84.3% in Portugal [38] and ethanolic extract at 5 mg/ml was 62.41% in Turkey. DPPH activity of MeOH extract of P. ostreatus was 29.0% at 2 mg/ml in Korea [37], 87.4% at 20 mg/ml in India [46] and 82.8% at 180 µg/ml in Turkey [7]. And also, fresh and steamed samples of P. ostreatus (1 mg/ml) showed the highest DPPH activity with 44.7% and 94.4%. It seemed that DPPH activities of L. deliciosus and P. ostreatus in this study were lower than those previous studies. The differences might be due to extraction methods, solvent types, used concentrations, localities and etc.
A good interaction between antioxidant capacity and reducing power is commonly accepted [47]. Antioxidants’ reduction capacities are usually associated with the presence of compounds which is breaking the free radical chain via contributing either electrons or hydrogen atom [21,48]. Antioxidants facilitate the conversion of Fe+3 to Fe+2. Higher the abs. value at the appropriate wavelength means the higher reducing power [21].
The results of the present study showed differences when compared with previous studies with regard to RP of L. deliciosus and P. ostreatus. RP of MeOH extract of L. deliciosus collected from Bragança region of Portugal was 2.41 at 50 mg/ml [38]; MeOH extract of P. ostreatus was 0.40 at 2 mg/ml in Korea [41], 1.97 at 10 mg/ml in India [46], 0.75 at 0.6 mg/ml in Black Sea region of Turkey [7], also ethanolic extract showed 1.367 absorbance at 10 mg/ml [49].
Based on these findings, L. deliciosus was higher RP in Bragança, Portugal [38] than that of Amanos Mountains, Turkey. Also, RP of P. ostreatus in this study was similar to Korean findings, whereas RP was lower than that of an Indian finding [46,49] and a Turkish study in the Black Sea region of Turkey [7]. It could be suggested that differences in RP might be due to different extracts, concentrations and localities.
NO is one of the free radicals synthesized by nitric oxide synthase from L-arginine [50] and can be toxic at high concentrations [51,52]. However, preventing the excessive production of nitric oxide is an important process [14]. In a previous report, it was reported that NOSA (%) of MeOH extract of Pleurotus squarrosulus and cultivated Pleurotus florida in India were 80 and 81.8 at 1 mg/ml, respectively [45,51], The findings of the present study seemed to lower than Pleurotus squarrosulus and cultivated Pleurotus florida in India.
Hydrogen peroxide occurring in tissues with oxidative processes is a relatively stable and type of the non-radical oxidant [18]. H2O2 is produced in the cytoplasm, plasma membrane and extracellular matrix of plants. In the cytoplasm, electron transport chain associated with the endoplasmic reticulum is known as the main source of H2O2 [53]. H2O2 accumulation in plant tissues has been acted as a signal between cells. Moreover, it also stimulates proteins related with stress responses as alternative oxidase catalase, peroxidase along with many genes [54,55]. It was reported that H2O2 damaged any tissue exposed to oxidative stress and caused the cancer [56]. Ozyurek et al. [18] determined that HPSA (%) of MeOH extract of Lactarius volemus, which purchased from local markets in different regions of Turkey, was 78.7% at 15 mg/ml. This assay depends on measuring the reducing capacity from Fe3+ to Fe2+ of materials and also widely accepted technique for antioxidant activity of different substances [23,57,58]. FRAP activities of Lactarius and Pleurotus genus have been reported in previous studies.
In Turkey, ethanolic extracts of L. deliciosus (Bolu-Turkey) were 0.229 at 250 μg/ml and 0.590 at 500 μg/ml [37]. In Thailand (Nakhon Ratchasima), FRAP activities of water and MeOH extracts of P. ostreatus were 4.38 and 1.61 at 20.0 mg/ml, respectively [43]. The significant correlations between antioxidant activities and antioxidant content were also reported in relation to phenolics, lycopene and carotenoids of tomato [59]. L. deliciosus and P. ostreatus collected from the natural habitats of the Amanos Mountains exerted notable antioxidant capacities; however, further studies are required to isolate and identify the specific compounds that are forming the antioxidant properties of the tested species.
The authors declare that there is no conflict of interest regarding the publication of this paper.
References![]()
- Halliwell B, Gutteridge J. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochemical journal, (1984); 219(1): 1-14.
- Chan MM-Y, Fong D, Ho C-T, Huang H-I. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochemical Pharmacology, (1997); 54(12): 1281-1286.
- Visioli F, Borsani L, Galli C. Diet and prevention of coronary heart disease: the potential role of phytochemicals. Cardiovascular Research, (2000); 47(3): 419-425.
- Barros L, Baptista P, Ferreira IC. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food and chemical Toxicology, (2007); 45(9): 1731-1737.
- Tsutomu N, Munetaka Y, Toshihiko O, Shunro K. Suppression of active oxygen-induced cytotoricity by flavonoids. Biochemical pharmacology, (1993); 45(1): 265-267.
- Halliwell B. Antioxidants in human health and disease. Annual review of nutrition, (1996); 16(1): 33-50.
- Elmastas M, Isildak O, Turkekul I, Temur N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. Journal of Food Composition and Analysis, (2007); 20(3-4): 337-345.
- Pekşen A, Karaca G. Macrofungi of Samsun province. Turkish Journal of Botany, (2003); 27(3): 173-184.
- Manzi P, Aguzzi A, Pizzoferrato L. Nutritional value of mushrooms widely consumed in Italy. Food chemistry, (2001); 73(3): 321-325.
- Lindequist U, Niedermeyer TH, Jülich WD. The pharmacological potential of mushrooms. Evidence-Based Complementary and Alternative Medicine, (2005); 2(3): 285-299.
- Palacios I, Lozano M, Moro C, D’arrigo M, Rostagno M, et al. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chemistry, (2011); 128(3): 674-678.
- Turkoglu A, Duru ME, Mercan N, Kivrak I, Gezer K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chemistry, (2007); 101(1): 267-273.
- Gursoy N, Sarikurkcu C, Cengiz M, Solak MH. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food and Chemical Toxicology, (2009); 47(9): 2381-2388.
- Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. Journal of agricultural and food chemistry, (1996); 44(3): 701-705.
- Guo T, Wei L, Sun J, Hou C-l, Fan L. Antioxidant activities of extract and fractions from Tuber indicum Cooke & Massee. Food Chemistry, (2011); 127(4): 1634-1640.
- Akata I, Ergönül B, Kalyoncu F. Chemical compositions and antioxidant activities of 16 wild edible mushroom species grown in Anatolia. International Journal of Pharmacology, (2012); 8(2): 134-138.
- Doğan HH. Evaluation of phenolic compounds, antioxidant activities and fatty acid composition of Amanita ovoidea (Bull.) Link. in Turkey. Journal of Food Composition and Analysis, (2013); 31(1): 87-93.
- Özyürek M, Bener M, Güçlü K, Apak R. Antioxidant/antiradical properties of microwave-assisted extracts of three wild edible mushrooms. Food chemistry, (2014); 157323-331.
- Muna GA, John M, Benson M, Ogoyi D. Antioxidant properties of cultivated edible mushroom (Agaricus bisporus) in Kenya. African Journal of Biotechnology, (2015); 14(16): 1401-1408.
- Mendil D, Uluözlü ÖD, Hasdemir E, Çaǧlar A. Determination of trace elements on some wild edible mushroom samples from Kastamonu, Turkey. Food Chemistry, (2004); 88(2): 281-285.
- Barros L, Ferreira M-J, Queiros B, Ferreira IC, Baptista P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food chemistry, (2007); 103(2): 413-419.
- Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction. Japanese Journal of Nutrition and Dietetics, (1986); 44(6): 307–315.
- Brand-Williams W, Cuvelier M-E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology, (1995); 28(1): 25-30.
- Benzie IF, Szeto Y. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. Journal of Agricultural and Food Chemistry, (1999); 47(2): 633-636.
- Ulukanli Z, Cigremis Y, Ilcim A. In vitro antimicrobial and antioxidant activity of acetone and methanol extracts from Thymus leucotrichius (Lamiaceae). European Review for Medical and Pharmacological Sciences, (2011); 15(6): 649-657.
- Ruch RJ, Cheng S-j, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, (1989); 10(6): 1003-1008.
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends in Plant Science, (1997); 2(4): 152-159.
- Hertog MG, Hollman PC, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. Journal of Agricultural and Food Chemistry, (1992); 40(12): 2379-2383.
- Frankel EN, Waterhouse AL, Teissedre PL. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. Journal of Agricultural and Food chemistry, (1995); 43(4): 890-894.
- Teissedre PL, Frankel EN, Waterhouse AL, Peleg H, German JB. Inhibition ofIn vitrohuman LDL oxidation by phenolic antioxidants from grapes and wines. Journal of the Science of Food and Agriculture, (1996); 70(1): 55-61.
- Donovan JL, Meyer AS, Waterhouse AL. Phenolic composition and antioxidant activity of prunes and prune juice (Prunus domestica). Journal of Agricultural and Food Chemistry, (1998); 46(4): 1247-1252.
- Stacewicz-Sapuntzakis M, Bowen PE, Hussain EA, Damayanti-Wood BI, Farnsworth NR. Chemical composition and potential health effects of prunes: a functional food? Critical Reviews in Food Science and Nutrition, (2001); 41(4): 251-286.
- Del Caro A, Piga A, Pinna I, Fenu PM, Agabbio M. Effect of drying conditions and storage period on polyphenolic content, antioxidant capacity, and ascorbic acid of prunes. Journal of Agricultural and Food Chemistry, (2004); 52(15): 4780-4784.
- Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutrition reviews, (1998); 56(2): 35-51.
- Rao A, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: a review. Nutrition Research, (1999); 19(2): 305-323.
- Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. The American journal of clinical nutrition, (1995); 62(6): 1315S-1321S.
- Orhan I, Üstün O. Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. Journal of Food Composition and Analysis, (2011); 24(3): 386-390.
- Ferreira IC, Baptista P, Vilas-Boas M, Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chemistry, (2007); 100(4): 1511-1516.
- Fernandes Â, Antonio AL, Barreira JC, Botelho ML, Oliveira MBP, et al. Effects of gamma irradiation on the chemical composition and antioxidant activity of Lactarius deliciosus L. wild edible mushroom. Food and Bioprocess Technology, (2013); 6(10): 2895-2903.
- Robaszkiewicz A, Bartosz G, Ławrynowicz M, Soszyński M. The Role of Polyphenols, β-carotene, and lycopene in the antioxidative action of the extracts of dried, edible mushrooms. Journal of nutrition and metabolism, (2010); 2010(1): 1-9.
- Kim JH, Kim SJ, Park HR, Choi JI, Ju YC, et al. The different antioxidant and anticancer activities depending on the color of oyster mushrooms. Journal of Medicinal Plants Research, (2009); 3(12): 1016-1020.
- Jeena GS, Punatha H, Prakash O, Chandra M, Kushwaha K. Study on in vitro antioxidant potential of some cultivated Pleurotus species (Oyster mushroom). Indian Journal of Natural Products and Resources (IJNPR)[Formerly Natural Product Radiance (NPR)], (2016); 5(1): 56-61.
- Chirinang P, Intarapichet K-O. Amino acids and antioxidant properties of the oyster mushrooms, Pleurotus ostreatus and Pleurotus sajor-caju. Science Asia, (2009); 35(2009): 326-331.
- Wettasinghe M, Shahidi F. Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago officinalis L.) seeds. Food Chemistry, (1999); 67(4): 399-414.
- Menaga D, Rajakumar S, Ayyasamy P. Free radical scavenging activity of methanolic extract of Pleurotus florida mushroom. International Journal of Pharmacy and Pharmaceutical Sciences, (2013); 5(Suppl 4): 601-606.
- Jeena GS, Punatha H, Prakash O, Chandra M, Kushwaha K. Study on in vitro antioxidant potential of some cultivated Pleurotus species (Oyster mushroom). Indian Journal of Natural Products and Resources, (2016); 5(1): 56-61.
- Tanaka M, Kuie C, Nagashima Y, al, Taguchi T. Application of antioxidative Maillard reaction products from histidine and glucose to sardine products. Nippon Suisan Gakkaishi, (1988); 54(8): 1409-1414.
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry, (1992); 40(6): 945-948.
- Jayakumar T, Thomas P, Geraldine P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innovative Food Science & Emerging Technologies, (2009); 10(2): 228-234.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biology and Medicine, (2010); 49(11): 1603-1616.
- Pal J, Ganguly S, Tahsin KS, Acharya K. In vitro free radical scavenging activity of wild edible mushroom, Pleurotus squarrosulus (Mont.) Singer. (2010).
- Lim H-W, Yoon J-H, Kim Y-S, Lee M-W, Park S-Y, et al. Free radical-scavenging and inhibition of nitric oxide production by four grades of pine mushroom (Tricholoma matsutake Sing.). Food Chemistry, (2007); 103(4): 1337-1342.
- Slesak I, Libik M, Karpinska B, Karpinski S, Miszalski Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. ACTA BIOCHIMICA POLONICA, (2007); 54(1): 39.
- Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. The Plant Cell, (1994); 6(1): 65-74.
- Vanlerberghe GC, Mclntosh L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiology, (1996); 111(2): 589-595.
- Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. The International Journal of Biochemistry & Cell Biology, (2000); 32(2): 157-170.
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. Journal of Agricultural and Food Chemistry, (2002); 50(3): 519-525.
- Wong C-C, Li H-B, Cheng K-W, Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chemistry, (2006); 97(4): 705-711.
- Ilahy R, Hdider C, Lenucci MS, Tlili I, Dalessandro G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. Journal of Food Composition and Analysis, (2011); 24(4-5): 588-595.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0