Review Article
3D-Bioprinting: A stepping stone towards enhanced medical approaches
Hajra Ashraf1,*, Bisma Meer1, Romasa Naz1, Aarooj Saeed1, Haleema Sadia2, Usmara Sajid3, Kanwal Nisar3, Zunaira Aslam3, Pervez Anwar3
Adv. life sci., vol. 5, no. 4, pp. 143-153, August 2018
*- Corresponding Author: Hajra Ashraf (Email: hajraashraf67@gmail.com)
Authors' Affiliations
2- Department of Biotechnology, Baluchistan University of Information Technology, Engineering and Management Sciences, Quetta, Pakistan
3- Department of Biochemistry and Molecular Biology, University of Sialkot, Punjab, Pakistan
Abstract![]()
Introduction
Methods
Discussion
Conclusion
References
Abstract
In the past few decades, tissue engineering has been seen unprecedented escalation driving the field of artificial tissue and organ construct and brought metamorphosis in regenerative medicine. Prime advancement has been attained through the expansion of novel biomanufacturing approaches to devise and convene cells in three dimensions to fabricate tissue contrive. Accompaniment manufacturing differently known as 3D bioprinting is leading prime innovation in a number of applications in life sciences such as tissue and organ construct, personalized drug dosing, cancer model and heart tissue engineering. Overall, this review summarizes most prevalent bioprinting technologies; including laser-based bioprinting, extrusion bioprinting, injection bioprinting, stereolithography as well as biomaterial such as bioink. It also explores 3D industries, approaches such as Biomimicry, autonomous self-assembly, mini tissues and biomedical applications. Existing challenges that impede clinical mileage of bioprinting are also discussed along with future prospective.
Keywords: Bioprinting, tissue engineering, tissue and organ construct, medicinal approaches
Bioprinting is a computational process for the assembly and designing of living and non-living materials and harvesting biologically engineered structures. It’s an alliance of technology, engineering, and sciences, using biomaterials and living cells for the generation of 2D and 3D complex biological constructs [1]. Most prevalent 3D bioprinting approaches are biomimicry, autonomous self-assembly, and mini tissue building blocks [2]. Biomimicry is the first approach in which skin and several organs are mimicked. Replication of organ occurs at micro-scale. The material which is constructed is similar to human organs [3,4].
In autonomous self-assembly approach, embryonic organ accounts further development and provide guides of replication. In the early stage of development, cells require adequate extracellular components, signaling, arrangement, and patterns homologous to organ [5]. Mini-tissue approach is evolved by a combination of biomimicry and autonomous self-assembly whose ultimate goal is to fabricate bigger tissues or organs from smaller components [3].
Current research emphasizes on new technologies in tissue engineering [6]. A noteworthy benefit is that bioprinting is complex and coherent designing either on the desktop or on an industrial scale. Comprehensively, 3D printing has been developed for a number of perks through operation of printing extracellular matrix or living cells components on the solid or liquid substrate. Three main modalities are offered by bioprinting which includes laser based [7], extrusion-based [8], and droplet-based [9]. Acellular techniques are also offered which usually comprises of stereolithography [10]. The most challenging aspect in this field is biomaterial development. Hydrogels and cell aggregates based biomaterials are also reported in the literature [1].
With notable benefits, propounded by bioprinting a wide range of applications including skin, heart and bone tissue engineering has been emerged [11]. Recent trends emboss regenerative medicine, drug discovery and tissue models [12], having the potential to substitute artifact. Currently, Bio-printing is widely used in many industries, such as the pharmaceutical industry, as well as in the ink-jet printers [13]. As an incipient and transpire field, several hurdles need to be overcome for triumphant transplantation of bioprinting particularly under consideration of technical, operative and regulatory matters [11,14].
In general, this review summarizes the data relevant to bioprinting, its approaches, technologies, and implementation on different fields including regenerative medicines, disease modeling and in tissue fabrication. In future 3D bio-printing will be a new frontier for the manufacturing of numerous materials used in surgery, medicine, cartilage replacement etc. [15].
Literature survey and selection criteria
A well-organized search was directed through Google scholar, PubMed Central and science direct, providing keywords “3D-Bioprinting a fate swap technology, “Approaches of Bioprinting”, “Bioinks for 3D bioprinting”, “Manufacturing technologies for 3D bioprinting”, “3D bioprinting medical application” etc. According to the particular contents further literature was screened and analyzed. In this study 80 research articles were selected to make a comprehensive review.
Bioprinting: A fate swap approach:
Bioprinting is an emerging technology, used for fabricating and manufacturing the artificial tissues. It’s an alliance of technology, engineering, and sciences, using biomaterials and living cells for the generation of 2D and 3D complex biological constructs. Organ transplantation is challenging owing to tissue rejection. Moreover, the incredible scarcity of human organ is accessible for transplant which later acts as a channel to develop tissue engineering. Tissue engineering is an integrative field used to restore damaged tissues or organs but has a number of limitations such as restricted cell density and accuracy in cell targeting. These obstructions are solved with the invention of the novel approach termed as bioprinting, playing an astonishing role in order to fabricate tissues or organs and their targeted placement in the body. It has also been exploited for skin purposes, by replacing damaged skin with 3D-printed skin [16]. The major applications of 3D bioprinting are described further in the review.
Approaches of bioprinting:
In order to print 3D organs with efficient structural and functional properties biomimicry, autonomous self-assembly, mini tissues approach or combinations of these are considered [17].
Biomimicry:
Biomimicry provides a solution of organ replacement by constructing organs and tissues that provide the same environment as the human body. This results in the construction of tissues or organs with constituents identical to tissue or organs of the body [18] Figure 1A. Several factors regarding this approach must be considered such as knowledge about microenvironment, soluble or insoluble factors gradient, arrangement of cell types, the composition of medium and nature of forces in the microenvironment. The success of this approach is reliable in several fields such as imaging, medicine, biophysics, cell biology and engineering [19,20].
Autonomous self-assembly:
Embryonic organ development plays an astonishing role in production of biological tissues [5]. Autonomous self-assembly relies on factors like structural and functional properties, localization as well as composition of tissues. For success of this approach, it is mandatory to have a complete knowledge of embryonic as well as organ developmental mechanism Figure 1B [21,22].
Mini tissues:
Mini tissue referred to as very small structural as well as a functional unit of tissue. It is a fundamental part of organ development [23]. Biomimicry and autonomous self-assembly are interrelated with mini tissues approach. Mini tissues and smaller building blocks result in the development of organ and tissue. To fabricate mini tissues into a larger one, self-assembly and rational design approach or combination are used Figure 1C [24]. It is based on two strategies. One is Cell spheres; which is self-assembled and arranged in a manner to construct microtissue followed by the biological organization. Other is the construction of tissue unit with high accuracy and resolution that are then assembled into micro tissue [3,22].
Imaging and designing:
It is an essential requirement to understand organization and composition of components for construction of tissues and organs which are heterogeneous in nature. To predict 3-D structure and function, medical imaging and designing play a remarkable role. For this two methods are commonly used which are given below:
- Magnetic resonance imaging (MRI)
- Computed tomography (CT) [25]
Computed tomography (CT):
It is used for diagnostic as well as therapeutic purposes. Its principle is based on the absorption rate of X-rays by tissues. To generate complex visual designs for tissue, computer-aided designs are also used. X-ray source surrounds the object and penetrates in the body which is then measured by sensors and results are recorded [26].
Magnetic resonance imaging (MRI):
In magnetic resonance imaging, an efficient spatial resolution is provided in soft tissues which rely on the strong magnetic field so; nuclei are easily aligned for imaging, in close proximity with high resolution. Any disturbances in energy states are sensed by the production of radio frequency signals. Iodine 30, barium 29 and metalloproteins 33 are also used to increase the contrast of biological structures such as blood vessels [27].
3D Bioprinting Techniques:
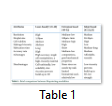
Bioprinting modalities being classified into three main groups comprising, laser, extrusion, and inkjet-based bioprinters. Despite these, acellular techniques are also available which are comprised of Stereolithography [1].
Laser-based Bioprinting:
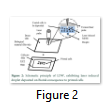
In 1990, Odde and Renn introduce laser based systems [7]. Laser-direct writing (LDW) is the most common method in laser bioprinting. The basic principle involves laser pulse, heating the slide comprising energy absorbing layer and cell’s solution. The laser pulse spawns sublimation or material’s vanishing, dislodging the cells on the opposite side. Thus, unequivocally depositing cells on the substrate Figure 2.
LDW comprises two methodologies, one is laser-induced forward transfer (LIFT) and the other is matrix-assisted pulse laser evaporation direct writing (MAPLE DW) [28]. MAPLE DW is just similar to LIFT, but uses low-intensity laser and permits small 3D structures e.g. keratinocytes/fibroblast and human mesenchymal stem cells (hMSC), fabrication through direct deposition of cells [29]. Cellular constructs, myoblasts, pulmonary artery and breast cancer cells are formed by MAPLE DW [30].
LDW is a nozzle-free technique, permits the use of high viscosity bio links. Barron et al. auspiciously printed mammalian cells on hydrogels [31]. Despite this system considers regulated printing for cells, there remain a few confinements that ought to be recognized. The heat produced from laser vitality or laser light might harm cells or influence the capability in final construct. In general, cell viability decline by laser-based bioprinting than that of inkjet-based bioprinting [32].
Extrusion based Bioprinting:
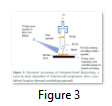
Extrusion or pressure based bioprinting commonly includes pressure or crew/plunger actuated dispensing fluid containing cells or biomaterials. The thin bioink is ideal for extrusion based bioprinting as it permits minimum resistance under glide but it chemically or bodily crosslinks successive layers [8].
Furthermore, shear forces must be considered for cell viability [33]. The key advantage, using a pneumatically driven device is numerous types and bio inks viscosities that are allotted by harmonizing the stress and valve gating time. During bioprinting, bio-ink is defrayed over deposited device under computational control, crosslinked via chemical, thermal transitions and light [32], ensuing in precise cells deposition encapsulated in cylindrical filaments of preferred 3D custom-shaped structures for diagrammatic illustration Figure 3.
For example, Yan et al. have deposited distinctive hepatocytes loaded with an extensive range of biocompatible hydrogels using extrusion-based bioprinting [34]. Even though, fabrication time lag to attain excessive-resolution, complicated structures are effectively tested by extrusion based bioprinting for the fabrication of clinically relevant scaffolds for tissue engineering [35].
Although, this era is taken into consideration because the maximum accessible approach for tissue and organ fabrication procedure, endures some limitations, consisting shear pressure and restrained fabric choice, for fast encapsulation of cells through gelation [36]. A higher allotting pressure avow ejecting viscous bioinks, but may boom the shear strain, which lessens cellular viability [37].
Inkjet based Bioprinting:
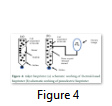
The roots of bioprinters are coined with inkjet printers, firstly they were used in offices then in personal computers in the 1980s. With a small lap, in 2000s traditional printer ink retrieved with cells. Cui et al. manifest a step-by-step approach to convert traditional printer to 3D bioprinter [38]. Inkjet bioprinting is a powerful method of depositing cell to create a 3D scaffold with fixed fluid volume using the software. It is the most popular method for cell-laden construct fabrication which mimics native tissues and organs [39]. Different inkjet printers are available, but thermal or piezoelectric drop-on are being in demand. It consists of the chamber of ink with a number of small nozzles along heating element. To generate ink-droplet, a current pulse is provided to the heating element. Thereupon a heating element flow of ink increases forcing a bubble to come out of a nozzle [40].
Conversely, piezoelectric inkjet uses a piezo crystal, when a current pulse is provided, crystal vibration cause ink out of the nozzle Figure 4 [40].
The biggest adverse effect happens inside the nozzle orifice by temperature. There is a need to mitigate this trouble [41]. Lorber et al. had been capable of efficiently printing retinal ganglion and glia cells harvested from the adult important anxious machine without inflicting a destructive impact on cellular viability [42]. Additionally, researchers have effectively tested a multi-head inkjet-based technique for bioprinting a couple of cell lines into heterogeneous scaffolds for tissue engineering [43,44]. A brief comparison of the formerly discussed Bioprinting modalities is given in Table 1.
Acellular Scaffold Fabrication:
The multi-axis positioning system is an additive method of 3D scaffold fabrication. Firstly, a model is created with computer-aided design (CAD), then it is conveyed to a file, making a 3D mesh in space as in stereolithography. “Slicer” a CAD program, translates data to a constructed model. These provide a high precision and versatility [54].
Stereolithography (SLA):
Stereolithography is an additive process that permits fabrication by CAD. Firstly, the 3D model is built with CAD, then SLA coordinates with model and constructs a 3D model, layer by layer usually ranging between 25-100µm [55]. Bottom-up and top-down are two approaches used in stereolithography, but top-down is mostly applied.
Commercially, a limited number of resins are available. The resin should readily coagulate by illuminating with light [56]. SLA is reported to fabricate molds implants for cranial surgery [57]. Overall, SLA is a versatile technique, owning precision for creating a tissue engineering scaffold, but, limited biocompatibility to one material is a big challenge faced in this method [6].
3D Bioprinting materials: Bioink
Most challenging demeanor in bioprinting is biomaterial, referred to as cell inclusions in biomaterial. Bio link is the principal unit in 3D construction. The physio-chemical parameters including swelling properties, rheological behavior, and gelatin kinetics must come in count as important factors for printability [58]. Two bioprinting materials reported in the literature are hydrogels and cell aggregates [59].
Hydrogels:
Hydrogels, in bioprinting, marked as the delivery vehicle of bio-ink. In biomanufacturing fields, hydrogels are widely used for cell encapsulation in a hydrated environment with computation. Hydrophilicity is the primary element to determine the biocompatibility of hydrogels, consequently making it appealing in the fabrication of tissue constructs [60].
In bioprinting, hydrogels are used as bioink because cells remain feasible, when encapsulated, including chondrocytes, hepatocytes, fibroblasts, smooth muscle cells, stem cells and adipocytes [61]. In TE, hydrogels either classified as natural or synthetic polymers. The natural polymers include alginate, fibrin, and chitosan while PEG (Polyethylene glycol and Pluronic are synthetic polymers [51].
All through bioprinting, a hydrogel with pendulous cells is processed into a 3D construct, successively fixed with gelation. Gelation is a crosslinking material, triggered by the physio-chemical process. Physical crosslinking is a reversible interaction that relies on meshes of excessive molecular polymer chains, ionic interactions and hydrogen bridges [47]. This type of crosslinking is compatible to living cells. The ionotropic gelatin between alginate anionic group (COO) with divalent meats ion (Ca2+) is one of its examples [47]. The poor mechanical properties, a key drawback in physical cross-linking. To overcome, additional cross-linking materials are used. Hydrogel viscosity is a second major aspect in bioprinting. Increased polymer concentration resulted in gel stiffness that minimizes cell mobility in an aqueous environment, thus forming a dense polymer network [62].
Cell aggregates:
During embryonic development, the cell undergoes self-organization, thus, forming tissues and organs evenly to a whole individual. Like this, bio printed cells undergo self-assembly resulting a final construct through cell-to-cell adhesion [63].
Cell aggregation success coined with the non-adhesive substrate, scaffold material and nutrients in a liquid medium. Collagen, a natural hydrogel used as a substrate for embryonic stem cell and bone marrow stem cells. Cell aggregates may be homogenous and heterogeneous comprising one type or more than one type of cells [64]. Bio link selection is a critical task in bioprinting and must be done according to construct nature and cell type.
Medical application of 3D Bioprinting:
3D bioprinting in the field of medical application broaden its spectrum and it’s expected to bring revolution in healthcare facilities. Medical application of 3D bioprinting covers a broad range of fields like tissue and organ regeneration, personalized drug dosing, cancer microenvironment and anatomical animals. Despite a number of benefits, 3D bioprinting still need a lot of research to overcome limitations associated with it.
Bioprinting tissues and organs or skin tissue engineering:
A condemnatory medical problem with the increase of diseases, age, accidents, and birth defects is tissue or organ failure. Its treatment predominantly depends upon organ transplant from a donor which may be living or deceased [65]. However, incredible scarcity of human organ is accessible for transplant. In 2017 almost 118,511 candidates were waiting for an organ. As of early 2016, 33,597 patients were on a waiting list. The major drawback of organ transplantation is its cost and the strenuous chore of finding a donor having similar genetic makeup [66].
This pitfall is abolished by using stem cells of the patient to build the whole organ in substitution of damage organ which would reduce the peril of tissue rejection or use of immunosuppressant. The possible solution for organ transplantation is to regenerate a person’s own organs which are done by tissue engineering. In conventional tissue engineering approach small tissue sample of patient is isolated, stem cells are extracted, mixed with growth factors and allowed to culture in lab by placing them onto a support called scaffolds from which the whole organ is regenerated. 3D bioprinting provide an additional salient benefit over conventional tissue engineering as having high control over concentration, volume, diameter and resolution of printed cells and producing them either layer by layer or directly into final 3D tissue-like structure Figure 5 [32]. Presently, ReCell® Spray-On Skin has been approved to be used for reconstructive burn by using patient own stem cells eradicating tissue rejection [26].
Personalized drug dosing:
The main objective of drug development is to lessen the menace of detrimental reactions and to increase its efficacy which can be attained through 3D printing to create personalized medicine [67]. The oral formulation is the most widely accepted dosage form because of pain avoidance, better acceptance, easy manufacturing and precise dosage. However, there is no feasible method accessible for personalized solid dosage form like tablets. Currently, tablets are prepared through processes such as milling, granulation, and mixing of constituents that are devised into a tablet by squeezing or shaping. Major hindrances during these steps are drug breakdown or swap resulting in difficulty with the formulation. Moreover, conventional processes are inappropriate to produce personalized medicine and limit the aptness to design dosage conceive with protracting strength, complex geometries and novel drug release profile [67]. Another perspective of 3D printing is to fabricate personalized medicine in new medication form such as pills that comprises multiple active components either as single or multilayer printed tablets. The Patients having multiple diseases could have liniments printed on one multidose form that is counterfeit and would revamp patient acquiescence. Amalgamate pharmacies could allocate 3D printed drugs by already intimating their customer to procure customized medicine [66].
Bioprinting the cancer microenvironment:
One of the prime roots of mortality in the world is ‘cancer’ approximately 8.8 million deaths were reported due to cancer in 2015 and this rate would be 70% over the next two decades. Therefore it is the need of the hour to comprehend cancer for its therapy. The most widespread types of cancer have the same characteristics and quirky microenvironment including both physical and chemicals signs like growth factor, interstitial pressure etc. At each stage of cancer this microenvironment is highly effective with distinguishing key factors, therefore its prime prerequisite to degrade and apprehend all elements involved in tumor metastasis and relocation [68]. To fully comprehend the complications of the cancer emergence, growth, progression, and metastasis human cancer models are required but they are not apprehended to imitate cancer in vivo microenvironment, therefore, 3D printing is used to engineered in vitro cancer microenvironment [69, 70]. Spheroid culture has been eventually used for 3D cancer models based on usage of 3D matrices either hydrogel or spongy supports called scaffold closely resemble human cancer microenvironment [71].
Heart tissue engineering:
Using a hydrogel which is a mixture of methacrylate hyaluronic acid and methacrylate gelatin a heart valve was formed by Duan et al through bioprinting. Firstly, the hydrogel was placed onto printed machine extended by nozzle on the platform and photo cross linked to from bioprinted heart valves having high viability. Thus bioprinting proved to be a stepping stone for heart tissue engineering [26,72].
3D-Bioprinting industries:
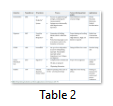
In commercial industry 3D, bio-printing is swiftly developing and manifesting the eminence of a mature market with mountainous potential. Several companies are already established the bio-printing technology and raising their finance through various paths. EnvisionTEC was founded in 2002, has high status for manufacturing the reliable manufacturing system. The product of EnvisionTEC is 3D-Bioplotter® System, which is used to organize the biomaterials using a computer. Organovo was developed in 2003 to print the organs, applications of drug discovery, and producing fully human tissues. Hydrogels are used as a temporary support. The printing process starts with bio-ink. Using hydrogel and bio-ink, the Blood vessel is designed layer-by-layer. (Courtesy of Organovo Inc.). GeSiM is another privately held company having the properly controlled software. The product of this company is Bio Scaffolder that is able to print scaffolds as well as living cells. It consists of a Z – stepper. Z-drives discretely prints enormous materials. It is a standard instrument platform which can fix up to four independent Z axes for relinquish materials. Cyfuse Biomedical K.K. Also develops 3D tissues and their Product is (Regenova®). They create 3D cellular structures by putting the cellular spheroids in needle arrays. It consists of three steps, in first step cell Spheroids are loaded and 3D data is prepared than in 2nd step 3Dprinting, spheroids are arranged into a 3D construct, and placed into a needle array in the third step that is maturing, 3D tissues are constructed into bio-reactors. [26,73-75] (Table 2).
Challenges and future prospective:
One of the major complications to construct human structure by 3D bioprinting is a dearth of coherency and mechanical strength to tolerate external stress and maintenance of shape after implantation. Significant research is conducted to intensify the resolution but despite fabrication of 3D structure, it’s not doable to maintain the actual inner structure, therefore, its need of the hour to further improve the 3D construct resolution [80].To make 3D construct survive prime issue to be resolved is vascularization of engineered tissue as these constructs require sufficient amount of nutrients and oxygen. Neo- vascularization is observed in case of 3D engineered tissue researches made their effort to make 3D printed structure fully vascularized however further advancement in this field is required [74]. Another obstacle in 3D bioprinting is processing time for fabrication. Extrusion bioprinting although increase the speed of construct fabrication but shear stress prompt cell damage so this pitfall must be solved before it is clinically implanted. As these challenges are resolved it will act as an outlook for the utility of bioprinting technologies on large scale as well as the integration of 3D printed cells and tissue will create a novel microenvironment for a number of life sciences application like cancer model, heart tissue engineering [81], personalized drug dosing and regenerative medicine [82].
In future, 3D bio-printing will be a new frontier for the manufacturing of numerous materials used in defense, and space industries as well as it would be an essential tool in the field of medical-biotechnology [16].
3D bioprinting has inimitable advantages towards organ development, personalized medicines and cancer microenvironment that are in preclinical trials. It’s an integrative technology that covers multiple disciplines such as biology, material, engineering, as well as medicine.3D printing is emanate discipline that accompaniment another imaging process in the implementation of composite procedures. Overall this review summarized accompaniment manufacturing process particularly known as 3D bioprinting and narrates a wide range of bioprinting techniques. It uses multiple approaches like Biomimicry, autonomous self-assembly and mini tissues that have depicted huge research on organ and tissue development as described in this review. Currently working 3D industries with bioprinting approaches are described as well. Besides all these, this paper gives the reader an outlook of current progression, limitations and future prospective of 3D bioprinting that will permit possible solution for wide range of bioprinting applications in a number of the field such as pharmaceutical and tissue and organ engineering.3D bioprinting in the field of tissue and organ transplantation have auspicious clinical prospective for organ regeneration.
Conflict of Interest Statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
References![]()
- Dababneh AB, Ozbolat IT. Bioprinting technology: a current state-of-the-art review. Journal of Manufacturing Science and Engineering, (2014); 136(6): 061016.
- Mandrycky C, Wang Z, Kim K, Kim D-H. 3D bioprinting for engineering complex tissues. Biotechnology advances, (2016); 34(4): 422-434.
- Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nature biotechnology, (2014); 32(8): 773-785.
- Biazar E, Najafi S M, Heidari K S, Yazdankhah M, Rafiei A, et al. 3D bio-printing technology for body tissues and organs regeneration. Journal of medical engineering & technology, (2018); 1-16.
- Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, et al. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication, (2010); 2(2): 022001.
- Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E. A review of three-dimensional printing in tissue engineering. Tissue Engineering Part B: Reviews, (2016); 22(4): 298-310.
- Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials, (2010); 31(28): 7250-7256.
- Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials, (2016); 76: 321-343.
- Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials, (2016); 102: 20-42.
- Skoog SA, Goering PL, Narayan RJ. Stereolithography in tissue engineering. Journal of Materials Science: Materials in Medicine, (2014); 25(3): 845-856.
- Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. Journal of translational medicine, (2016); 14(1): 271.
- Gao G, Cui X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnology letters, (2016); 38(2): 203-211.
- Daly R, Harrington TS, Martin GD, Hutchings IM. Inkjet printing for pharmaceutics–a review of research and manufacturing. International journal of pharmaceutics, (2015); 494(2): 554-567.
- Hong N, Yang GH, Lee J, Kim G. 3D bioprinting and its in vivo applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials, (2017); 10(2): 444-459.
- Gu Q, Hao J, Lu Y, Wang L, Wallace GG, et al. Three-dimensional bio-printing. Science China Life Sciences, (2015); 58(5): 411.
- Seol Y-J, Kang H-W, Lee SJ, Atala A, Yoo JJ. Bioprinting technology and its applications. European Journal of Cardio-Thoracic Surgery, (2014); 46(3): 342-348.
- Huang Y, Zhang XF, Gao G, Yonezawa T, Cui X. 3D bioprinting and the current applications in tissue engineering. Biotechnology Journal, (2017); 12(8): 734
- Pati F, Gantelius J, Svahn HA. 3D bioprinting of tissue/organ models. Angewandte Chemie International Edition, (2016); 55(15): 4650-4665.
- Abdullaeva Z. Tissue Engineering, Scaffolds, and 3D Bioprinting. Nano‐and Biomaterials: Compounds, Properties, Characterization, and Applications, (2017); 259-282.
- Boura K. 3-D Bioprinting. Accessed online at https://slideplayer.com/slide/12567014/.
- Yu Y, Moncal KK, Li J, Peng W, Rivero I, et al. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Scientific reports, (2016); 628714.
- Ozbolat I, Gudapati H. A review on design for bioprinting. Bioprinting, (2016); 31-14.
- Baker E, Evans T, Gould D, Hull W, Keegan H. A manual of parasitic mites of medical or economic importance. National Pest Control Association. Inc, New York, New York, (1956); 74-78.
- Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends in biotechnology, (2015); 33(7): 395-400.
- Mironov V, Reis N, Derby B. Bioprinting: a beginning. Tissue engineering, (2006); 12(4): 631-634.
- Chua CK, Yeong WY. (2015). Bioprinting Principles and Applications. In: World Scientific Series in 3D Printing. World Scientific, (1); pp: 2-26.
- Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, et al. In vivo bioprinting for computer-and robotic-assisted medical intervention: preliminary study in mice. Biofabrication, (2010); 2(1): 014101.
- Ringeisen BR, Othon CM, Barron JA, Young D, Spargo BJ. Jet‐based methods to print living cells. Biotechnology journal, (2006); 1(9): 930-948.
- Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, et al. Laser printing of skin cells and human stem cells. Tissue Engineering Part C: Methods, (2009); 16(5): 847-854.
- Schiele NR, Koppes RA, Corr DT, Ellison KS, Thompson DM, et al. Laser direct writing of combinatorial libraries of idealized cellular constructs: biomedical applications. Applied Surface Science, (2009); 255(10): 5444-5447.
- Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta biomaterialia, (2010); 6(7): 2494-2500.
- Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Transactions on Biomedical Engineering, (2013); 60(3): 691-699.
- Tan EYS, Yeong WY. Concentric bioprinting of alginate-based tubular constructs using multi-nozzle extrusion-based technique. International Journal of Bioprinting, (2015); 1(1): 49-56.
- Yan Y, Wang X, Pan Y, Liu H, Cheng J, et al. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials, (2005); 26(29): 5864-5871.
- Chang R, Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Engineering Part A, (2008); 14(1): 41-48.
- Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials, (2012); 33(26): 6020-6041.
- Fedorovich NE, De Wijn JR, Verbout AJ, Alblas J, Dhert WJ. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Engineering Part A, (2008); 14(1): 127-133.
- Xu T, Zhao W, Zhu J-M, Albanna MZ, Yoo JJ, et al. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials, (2013); 34(1): 130-139.
- Nakamura M, Nishiyama Y, Henmi C, Yamaguchi K, Mochizuki S, et al. Inkjet bioprinting as an effective tool for tissue fabrication. 2006; pp. 89-92. Society for Imaging Science and Technology.
- Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert opinion on biological therapy, (2007); 7(8): 1123-1127.
- Kesti M, Müller M, Becher J, Schnabelrauch M, D’Este M, et al. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta biomaterialia, (2015); 11: 162-172.
- Lorber B, Hsiao W-K, Hutchings IM, Martin KR. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication, (2013); 6(1): 015001.
- Ker ED, Chu B, Phillippi JA, Gharaibeh B, Huard J, et al. Engineering spatial control of multiple differentiation fates within a stem cell population. Biomaterials, (2011); 32(13): 3413-3422.
- Cooper GM, Miller ED, DeCesare GE, Usas A, Lensie EL, et al. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue Engineering Part A, (2010); 16(5): 1749-1759.
- Odde DJ, Renn MJ. Laser-guided direct writing for applications in biotechnology. Trends in biotechnology, (1999); 17(10): 385-389.
- Barron JA, Ringeisen BR, Kim H, Spargo BJ, Chrisey DB. Application of laser printing to mammalian cells. Thin Solid Films, (2004); 453: 383-387.
- Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, et al. 25th anniversary article: engineering hydrogels for biofabrication. Advanced materials, (2013); 25(36): 5011-5028.
- Raof NA, Schiele NR, Xie Y, Chrisey DB, Corr DT. The maintenance of pluripotency following laser direct-write of mouse embryonic stem cells. Biomaterials, (2011); 32(7): 1802-1808.
- Smith CM, Stone AL, Parkhill RL, Stewart RL, Simpkins MW, et al. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue engineering, (2004); 10(9-10): 1566-1576.
- Moon S, Hasan SK, Song YS, Xu F, Keles HO, et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Engineering Part C: Methods, (2009); 16(1): 157-166.
- Censi R, Schuurman W, Malda J, Di Dato G, Burgisser PE, et al. A printable photopolymerizable thermosensitive p (HPMAm‐lactate)‐PEG hydrogel for tissue engineering. Advanced Functional Materials, (2011); 21(10): 1833-1842.
- Pescosolido L, Schuurman W, Malda J, Matricardi P, Alhaique F, et al. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules, (2011); 12(5): 1831-1838.
- Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, et al. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue engineering, (2005); 11(11-12): 1658-1666.
- Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials, (2000); 21(24): 2529-2543.
- Melchels FP, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials, (2010); 31(24): 6121-6130.
- Bártolo PJ Stereolithography: materials, processes and applications. 2011; Springer Science & Business Media.
- Sinn DP, Cillo Jr JE, Miles BA. Stereolithography for craniofacial surgery. Journal of Craniofacial Surgery, (2006); 17(5): 869-875.
- Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, et al. Bioink properties before, during and after 3D bioprinting. Biofabrication, (2016); 8(3): 032002.
- Duan B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Annals of biomedical engineering, (2017); 45(1): 195-209.
- Zhu W, Ma X, Gou M, Mei D, Zhang K, et al. 3D printing of functional biomaterials for tissue engineering. Current opinion in biotechnology, (2016); 40: 103-112.
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature, (2009); 462(7272): 433-441.
- Khalil S, Sun W. Bioprinting endothelial cells with alginate for 3D tissue constructs. Journal of biomechanical engineering, (2009); 131(11): 111002.
- Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, et al. Organ printing: tissue spheroids as building blocks. Biomaterials, (2009); 30(12): 2164-2174.
- Jakab K, Norotte C, Damon B, Marga F, Neagu A, et al. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Engineering Part A, (2008); 14(3): 413-421.
- Cui X, Boland T, D D'Lima D, K Lotz M. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent patents on drug delivery & formulation, (2012); 6(2): 149-155.
- Ventola CL. Medical applications for 3D printing: current and projected uses. Pharmacy and Therapeutics, (2014); 39(10): 704.
- Banks J. Adding value in additive manufacturing: Researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE pulse, (2013); 4(6): 22-26.
- Cao Y. Tumor angiogenesis and therapy. Biomedicine & pharmacotherapy, (2005); 59S340-S343.
- Alemany-Ribes M, Semino CE. Bioengineering 3D environments for cancer models. Advanced drug delivery reviews, (2014); 79: 40-49.
- Unger C, Kramer N, Walzl A, Scherzer M, Hengstschläger M, et al. Modeling human carcinomas: physiologically relevant 3D models to improve anti-cancer drug development. Advanced drug delivery reviews, (2014); 79: 50-67.
- Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, et al. Engineering tumors with 3D scaffolds. Nature methods, (2007); 4(10): 855-860.
- Duan B, Kapetanovic E, Hockaday LA, Butcher JT. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta biomaterialia, (2014); 10(5): 1836-1846.
- Hockaday L. 3D bioprinting: a deliberate business. Genetic Engineering & Biotechnology News. (2014); 35(1): 1089.
- Arslan-Yildiz A, El Assal R, Chen P, Guven S, Inci F, et al. Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication, (2016); 8(1): 014103.
- Doyle K. Bioprinting: from patches to parts. Genetic Engineering & Biotechnology News. (2014); 34(10): 1089.
- Coatney S, Gandhi B, Park BS, Dzilno D, Tapia EM, et al. 3D bio-printing. Fung Institute for Engineering Leadership, University of California at Berkeley, (2013).
- Rezende RA, Pereira F, Brakke K, Mombach J, Kasyanov V, et al. In silico 3D bioprinting: computer modeling and simulation of organ printing. Tissue Enginnering Part A, (2014); 2025-25.
- Collins SF. Bioprinting is changing regenerative medicine forever. Stem cells and development, (2014); 23(S1): 79-82.
- Chang SL, Chen JK. 3D bio-printing in medical treatment: A technology acceptance model; 2016. IEEE. pp. 3149-3154.
- Dado D, Levenberg S. Cell–scaffold mechanical interplay within engineered tissue; 2009. Elsevier. pp. 656-664.
- Rizvi I, Gurkan UA, Tasoglu S, Alagic N, Celli JP, et al. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proceedings of the National Academy of Sciences, (2013); 110(22): E1974-E1983.
- Guven S, Chen P, Inci F, Tasoglu S, Erkmen B, et al. Multiscale assembly for tissue engineering and regenerative medicine. Trends in biotechnology, (2015); 33(5): 269-279.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0