Full Length Research Article
Expression level of serum Interleukin-37 in Rheumatoid Arthritis patients and its correlation with Disease Activity Score
Nimrah Akram, Arshad Jamal, Sajjad Ullah, Ahmed Bilal Waqar*, Khurshid Iqbal
Adv. life sci., vol. 5, no. 4, pp. 159-165, August 2018
*- Corresponding Author: Ahmed Bilal Waqar (Email: drabwaqar@yahoo.com)
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Interleukin-37 (IL-37) is a member of IL-1 cytokine family. IL-37 immunosuppresses the pathogenesis of rheumatoid arthritis via down-regulating pro-inflammatory cytokines. The aim of the current study was to evaluate the expression level of IL-37 in rheumatoid arthritis (RA) patients and its correlation with the disease activity score in 28 joints (DAS-28).
Methods: In the current study, forty-six RA patients, having a ratio of 19 males and 27 females, and twenty healthy controls (11 males and 9 females) were included. DAS-28 was measured on the basis of patients’ clinical observations of the tender and swollen joints, physical examination and erythrocyte sedimentation rate (ESR). ESR was measured according to the Westergren method. Serum IL-37 level was measured by ELISA. Depending upon the DAS28 calculations the patients were divided in four groups as; 19 in remission, 6 had mild disease activity, 6 were in moderate state and 15 patients were found with severe disease activity.
Results: Serum IL-37 levels were found markedly raised in RA patients (mean = 862.6) than in healthy individuals (mean ± SD = 4.4 ± 1.74 pg/ml). Further, our results suggest that level of IL-37 increased significantly from mild (mean ± SD = 829.17 ± 61.40 pg/ml) to moderate (mean ± SD = 1307.5 ± 165.1 pg/ml) and severe (mean ± SD = 1607 ± 86.8 pg/ml) disease prognosis.
Conclusion: Thus we conclude, IL-37 has a positive correlation with DAS28 and thus has a potential role in RA pathogenesis.
Keywords: Interleukin 37, rheumatoid arthritis, autoimmune disorder, inflammation, disease activity score
Rheumatoid arthritis (RA) is a systemic, chronic inflammatory autoimmune disorder characterized by immune cells infiltration, inflammatory joints and synovial hyperplasia [1]. It primarily affects the peripheral joints. The accurate pathogenesis and etiology of RA remain elusive, though cytokines are considered to play a central role in disease progression through inflammation and articular destruction [2]. An imbalance between all pro inflammatory and anti-inflammatory cytokines leads to the progression of disease symptoms [3]. RA causes swelling of joints, tenderness of joints and also the destruction of synovial joints that leads to severe disability and premature fatality [4]. Rheumatologists don’t understand the obvious cause of RA development but the most possible risk factors include genetic background, hormones, environmental factors, smoking [5], microbes in the bowel and obesity. Middle aged mostly women are frequently affected as compared to men [6]. Estimated RA prevalence is recorded as 1% worldwide [1]. The pathogenesis of RA is a multi-step process and is divided as autoimmunity, systemic inflammation and then leading to bone destruction. Certain cells of the immune system start attacking the healthy tissues causing inflammation specifically in the synovium, the tissue lining the joints. Due to the detection of auto-antibodies including rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs), RA is considered to be an autoimmune disease [7]. RF is not considered as RA specific as it may be detected in hepatitis C viral infection and older people whereas ACPA is RA specific and plays significant role in RA pathogenesis [8]. RF and ACPAs, cause an immune deregulation in RA patients. Due to the localization of inflammatory cells in the synovial lining of the joints, thickening and hyperplasia develop [9].
Interleukin 37 (IL-37) has been included in interleukin 1 cytokine family (IL1F7) [10,11]. IL-37 is an anti-inflammatory cytokine and has a potential role in various inflammatory diseases like ankylosing spondylitis [12], graves’ disease [13], rheumatoid arthritis [14], systemic lupus erythematosus (SLE) [15] and inflammatory bowel disease [16]. High levels of IL-37 are detected in the serum and synovial fluid of all RA patients with different ranges depending upon the severity of the disease. Elevated IL-37 level inhibits pro inflammatory cytokines synthesis [17]. IL-37 has anti-inflammatory activity in both innate and acquired immunity. It plays a significant role in the control of RA pathogenesis by suppressing inflammation and innate immunity. Injecting IL-37 is considered to be the novel RA therapy. It functions as a down regulator of local and systemic inflammations [18]. It is expressed in various cells, tissues and organs including plasma cells, monocytes, epithelial cells, macrophages, dendritic cells, thymus, testis and uterus [19]. Its specific role is to down regulate inflammation, innate immunity and adaptive immunity. Lipo-polysaccharides (LPS) may also be induced for the maintenance of IL-37 expression levels in RA patients so that it might not be completely diminished. Treatment with LPS is found very helpful in the improvement of lungs and kidneys functioning and also in the prevention of liver damage [20].
Disease Activity Score (DAS) is a scoring strategy implies for the evaluation of disease severity of RA [21]. DAS28 includes 28 joints to be examined. DAS28 is calculated on the basis of number of all swollen and tender joints [21], visual assessment of patients’ disease severity and erythrocyte sedimentation rate (ESR). ESR is required for indicating the degree of inflammation. RA treatment therapies can slow down the joint deterioration and inflammation leading to disability. On the basis of DAS28, RA patients can be categorized in four levels; in remission DAS28<2.6, in low disease activity DAS28≤3.2, in moderate disease activity DAS≤5.1 and in high or severe disease activity DAS28>5.1 [21]. DAS28 evaluation every four weeks can help your doctors and you to decide whether your treatment needs any changes for further improvements [22]. Disease activity scoring was made to evaluate the disease severity of the patients clinically to minimize other related complications and also to modify the treatment therapy according to the severity level of the disease. Originally DAS was developed to assess the disease onset in early affected RA patients. Newly developed indices for DAS evaluation are to investigate the severity of patients in early stage as well as in the severe diseased. DAS28 is now considered and more preferable and simple method for the evaluation of RA subjects.
The current study demonstrates, the serum level of IL-37 in RA patients and its correlation with DAS28 score in inactive and severely diseased patients.
Patients and clinical evaluations:
Forty-six RA patients from Sharif Medical City hospital Lahore-Pakistan were included in this study. Patients fulfilling the Rheumatology criteria of American College of Rheumatology were selected. In addition, twenty healthy individuals of more than 30 years of age were taken as controls. This study was first approved by the institutional Ethical Review Board (ERB) of Imperial College of Business Sciences (ICBS), Lahore-Pakistan. Informed consent was signed from all the study participants and counseling, related to our further investigations, was done before subjecting the patients.
All patients were presented with varying symptoms and complaints. The total count of tender and swollen joints of each patient was calculated on DAS28 score; ESR was tested on immediate basis and disease severity was assessed for DAS28 calculations. DAS28 grouping was done according to the disease severity of the patients. On the basis of DAS28 evaluations, all patients (n=46) were divided in four groups: remission (DAS28≤2.6), mild (2.6<DAS28≤3.2), moderate (3.2<DAS28≤5.1) and severe (DAS28>5.1). While examining the patient clinically, blood samples were collected for ESR and interleukin level measurement.
Serum IL-37 level measurement:
Clotted blood samples were centrifuged at 1300 g (RCF) for 10 minutes and serum was separated in specifically labeled serum cups. All the samples were stored at freezing temperature until analysis. Serum IL-37 level of all the samples was detected via enzyme linked immunosorbent assay (ELISA) (Cusabio CSB E16185h). IL-37 specific antibody was pre-coated on microplate. 100 μl standard and samples were loaded in allocated wells and incubated for 2 hours at 37oC. Wells were washed twice to remove unbound proteins. 100 μl of biotin antibody was added and plate was wrapped in aluminum foil and incubated at 37oC for 1 hour. After incubation and washing, 100 μl horse radish peroxidase (HRP) avidin solution was added and incubated for one hour. Wells were again washed; 90 μl TMB substrate was added and kept in dark for 30 minutes. Color reaction developed was directly proportional to the presence of IL-37 levels in it. After incubation, 50 μl of stop solution was added. OD values of the microplate were recorded at 450nm.
Statistical analysis:
Prism Graph Pad was used to analyze the data. Unpaired t-test was employed to determine statistically significant difference among various diseased groups. The difference was significant when p value was less than 0.05. The correlation between IL-37 level and DAS28 was analyzed by Pearson’s test. Significant correlation was evaluated as P<0.05.
Clinical assessment of research subjects:
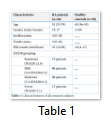
For the evaluation of serum IL-37 expression levels, forty-six RA patients (19 males and 27 females) average age of 51 years (ranged from 33 to 79 years) and twenty healthy controls (11 males and 09 females) average age of 43 years (ranged from 36 to 53 years) were included. DAS28 results were analyzed on the basis of tender and swollen joints count, ESR and the physical severity of the disease regarding patients’ pain (table 1). On the basis of DAS28 results, 46 RA patients were divided; 19 remission, 6 mild, 6 moderate and 15 in severe disease activity group.
Gender ratio:
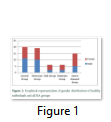
Among healthy individuals, 11 were males and 9 were females. Accordingly the gender ratio of RA patients included, 19 patients were male and 27 were of female gender. RA remission patients were 10 males and 9 females. Out of six patients from mild group, 2 were males and 4 were females. Moderate group patients included 2 males and 4 females. In severe diseased patients, 5 were males and 10 were females. (Figure 1)
Serum IL-37 measurement:
The level of IL-37 in healthy controls’ serum was remarkably low, mean ± SD = 4.4 ± 1.7 pg/ml. On contrary, it was markedly raised in RA patients with mean = 862.6 pg/ml. Interestingly, IL-37 level increased significantly with disease projection from mild to moderate and severe conditions. Concisely, in remission patients the IL-37 level (mean ± SD = 145.16 ± 48.39 pg/ml) was lowest among the four categories. Additionally, the level of IL-37 in the mild, moderate and severe patients was observed to be [mean ± SD] 829.17 ± 61.40 pg/ml, 1307.5 ± 165.1 pg/ml and 1607 ± 86.8 pg/ml respectively (Table 2) (Figure 2).
Serum IL-37 level and DAS28 correlation:
Serum IL-37 level was found markedly low in all healthy controls having no chronic or inflammatory diseases, while it was found strongly elevated in all RA patients. Interleukin levels and disease severity was correlated. The P value of correlation between serum IL-37 patients and DAS28 of RA subjects was significant (P<0.05) (Table 3). Our results demonstrated that the correlation between remission (-0.018), mild (-0.077) andmoderate (-0.307) patient groups and IL37 was negative. However, the IL37 level was positively correlated with severe patients (0.168).
Tables & Figures
RA is a systemic, chronic inflammatory and autoimmune disease having cell infiltration, uncontrolled synovial cells proliferation and cartilage destruction [23]. Numerous studies have reported the elevated levels of pro inflammatory cytokines such as TNF, IL-1 and IL-6 in RA patients. Inhibition of these inflammatory cytokines is considered to be the treatment goal for such patients [24]. Most of the pro inflammatory cytokines have a potential role in the pathogenesis of the disease, as their serum and synovial fluid levels are significantly raised [4,25]. In various inflammatory autoimmune diseases IL-37, an anti-inflammatory cytokine, levels increases with the increase of inflammatory cytokines [17]. Thus IL-37 has been emerged as a novel anti-inflammatory cytokines that possesses extra- and intracellular properties. It suppresses inflammation and the innate immune responses [26]. Its potential protective role is noticed in several animal models such LPS induced shock [27], dextran sulphate sodium induced colitis [28], insulin resistance and obesity induced inflammation, hepatitis B virus infection [29], Aspergillus infection [30], Graves’ disease and concanavalin A-induced hepatitis. On the other hand, down-regulation of IL-37 expression is observed in Bechet’s disease [31], invertebral disc regeneration [32] and Vogt-Koyanagi-Harada disease [33].
In the current study we are reporting the correlation of IL-37 level with the prognosis of RA in Pakistani population, which was not reported before. Our data demonstrated the increased serum IL-37 level all RA patients (mean = 862.6 pg/ml) and was reduced considerably in healthy individuals (mean ± SD was 4.4 ± 1.7 pg/ml). Published studies suggest the significant relation between the IL-37 level and the disease activity and pro-inflammatory cytokines production. Yang L et al, reported a positive correlation of elevated IL-37 with pro-inflammatory cytokines and disease activity in all RA patients [8, 13]. It has been reported that immune histochemical staining of synovial lining of RA patients have elevated IL-37 level as compared to healthy controls [20]. The current study demonstrated that serum IL-37 level was markedly low in remission RA (mean ± SD = 145.16 ± 48.39 pg/ml) patients than severely diseased active RA patients (1607 ± 86.8 pg/ml) suggesting that remission RA patients had low pro- inflammatory cytokines and decreased symptoms and joint complications. This suggests that elevated production of pro-inflammatory cytokines might have triggered the elevated levels of IL-37 [3]. Both pro- and anti-inflammatory cytokines levels are known to be raised in RA patients. Sequentially, the presence of pro-inflammatory cytokines may up regulate the production of IL-37 [17] and IL-37 may down regulate the excessive level of pro- inflammatory cytokines [19]. Although the expression level of IL-37 is high in severe active RA patients, however as compared to the level of pro-inflammatory cytokines it is still low, and one of the most probable reasons of disease progression in such patients [34]. Thus the uncontrolled inflammation in RA patients is due to inadequate working of antagonistic cytokines, specifically IL-37 [19]. A previous study has shown that in all the inactive remission state patients there was significantly decreased level of serum IL-37 and was majorly regulated by pro-inflammatory cytokines [3]. Based on our results and previously reported results, it is hypothesized that IL-37 produced due to activated immune responses has a vital role in RA pathogenesis, and promote inflammation via suppression of pro-inflammatory cytokines.
In current study, we demonstrated that serum IL-37 level had positive correlation with DAS28 in RA patients (p < 0.05). Consistent with the results, a positive correlation of IL-37 and DAS28 was previously recorded in China among 50 RA patients [3] and in Changhai among 34 RA patients [19]. Thus, IL-37 is the most probable biomarker of RA diagnostics, disease activity evaluation and remedial response observations [35]. The results of the present study demonstrated that women were more frequently affected by RA as compared to men (27 females and 19 males). It was reported in Oslo that the prevalence of RA in females was two times greater than in men [36]. Jacquiline has also reported that females are affected two to three times greater as compared to males. Moreover it has been noticed that in majority of the autoimmune diseases females are predominantly affected [37].
In conclusion, the level of IL-37 in serum was markedly high in RA patients, in Pakistani population, specifically in the severe diseased patients. Positive correlation was demonstrated between IL-37 levels and disease severity (with P value less than 0.05). The limitation of the current study is that, we could not investigate the serum level of other anti and pro-inflammatory cytokines and their correlation with the level of IL-37. However, the current data highlights the fact that increased serum IL-37 has a potential anti-inflammatory role in RA pathogenesis.
Conflict of interest
Authors declare that there is no conflict of interest for publishing this study.
The Study was supported by Faculty of Health and Allied Sciences, Imperial College of Business Studies.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature, (2003); 423(6937): 356–61.
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. Journal of Clinical Investigation. 2008;118(11):3537–45.
- Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan YX, et al. Plasma levels of IL-37 and correlation with TNF-a, IL-17A, and Disease activity during DMARD treatment of rheumatoid arthritis. PLoS One. 2014;9(5).
- Wolfe F. The natural history of rheumatoid arthritis. Journal of Rheumatology. (1996) ; 44:13–22.
- Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet, (2017); 12: 376(9746):1094–108.
- Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis & Rheumatism, (2011); 63(3): 633–9.
- Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet, (2016); 370(9602): 1861–74.
- Balsa A, Cabezón A, Orozco G, Cobo T, Miranda-Carus E, et al. Influence of HLA DRB1 alleles in the susceptibility of rheumatoid arthritis and the regulation of antibodies against citrullinated proteins and rheumatoid factor. Arthritis Research & Therapy, (2010); 12(2): R62.
- Tarner IH, Harle P, Muller-Ladner U, Gay RE, Gay S. The different stages of synovitis: acute vs chronic, early vs late and non-erosive vs erosive. Best Practice & Research: Clinical Rheumatology, (2005); 19(1): 19–35.
- Busfield SJ, Comrack CA, Yu G, Chickering TW, Smutko JS, et al. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics, (2000); 66(2): 213–6.
- Smith DE, Renshaw BR, Ketchem RR, Kubin M, Garka KE, et al. Four new members expand the interleukin-1 superfamily. The Journal of Biological Chemistry, (2000); 275(2): 1169–75.
- Chen B, Huang K, Ye L, Li Y, Zhang J, Zhang J, et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. Journal of Translational Medicine, (2015); 13: 36.
- Xia S, Wei J, Wang J, Sun H, Zheng W, Li Y, et al. A requirement of dendritic cell-derived interleukin-27 for the tumor infiltration of regulatory T cells. Journal of Leukocyte Biology, (2014); 95(5): 733–42.
- Yang L, Zhang J, Tao J, Lu T. Elevated serum levels of Interleukin-37 are associated with inflammatory cytokines and disease activity in rheumatoid arthritis. Acta Pathologica, Microbiologica et Immunologica Scandinavica, (2015); 123(12): 1025–31.
- Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: its correlation with disease activity. Journal of Translational Medicine, (2014); 12: 69.
- Weidlich S, Bulau A-M, Schwerd T, Althans J, Kappler R, Koletzko S, et al. Intestinal expression of the anti-inflammatory interleukin-1 homologue IL-37 in pediatric inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition, (2014); 59(2): e18-26.
- Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, et al. IL-37: A new anti-inflammatory cytokine of the IL-1 family. European Cytokine Network, (2011); 22(3): 127–47.
- Xu S, Li W, Tong Y, Dong N, Sheng Z, Yao Y. Expression of IL-37 contributes to the immunosuppressive property of human CD4 + CD25 + regulatory T cells. Scientific Reports, (2015); 5: 14478.
- Xia T, Zheng XF, Qian BH, Fang H, Wang JJ, et al. Plasma interleukin-37 is elevated in patients with rheumatoid arthritis: Its correlation with disease activity and Th1/Th2/Th17-related cytokines. Disease Markers, (2015); 2015: 795043.
- Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, et al. IL-37 is a fundamental inhibitor of innate immunity. Nature Immunology, (2010); 11(11): 1014–22.
- Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, et al. Disease Activity Score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients. Annals of the Rheumatic Diseases, (2007); 66(9): 1221–6.
- Fransen J, Stucki G, van Riel PLCM. Rheumatoid arthritis measures: Disease Activity Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI). Arthritis & Rheumatology, (2003); 49(S5): S214–24.
- Biswas S, Sharma S, Saroha A, Bhakuni DS, Malhotra R, et al. Identification of novel autoantigen in the synovial fluid of rheumatoid arthritis patients using an immunoproteomics approach. PLoS One. 2013;8(2): e56246.
- Hurkmans E, van der Giesen FJ, Vliet Vlieland TP, Schoones J, Van den Ende ECHM. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. The Cochrane Database of Systematic Reviews, (2009); (4): CD006853.
- Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis & Rheumatology, (2006); 54(3): 692–701.
- Bulau AM, Nold MF, Li S, Nold-Petry CA, Fink M, et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America, (2014); 111(7): 2650–5.
- Feldmann M, Maini RN. The role of cytokines in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford), (1999); 38(2): 3-7.
- Mcnamee EN, Masterson JC, Jedlicka P, Mcmanus M, Grenz A, et al. Interleukin 37 expression protects mice from colitis. Proceedings of the National Academy of Sciences of the United States of America, (2011); 108(40): 16711–6.
- Li C, Ji H, Cai Y, Ayana DA, Lv P, et al. Serum interleukin-37 concentrations and HBeAg seroconversion in chronic HBV patients during telbivudine treatment. Journal of Interferon & Cytokine Research, (2013); 33(10):612–8.
- Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG, et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLOS Pathogens, (2014); 10(11): e1004462.
- Ye Z, Wang C, Kijlstra A, Zhou X, Yang P. A possible role for interleukin 37 in the pathogenesis of Behcet’s disease. Current Molecular Medicine, (2014); 14(4): 535–42.
- Wan ZY, Sun Z, Song F, Chen YF, Zhang WL, Wang HQ, et al. Downregulated interleukin 37 expression associated with aggravation of intervertebral disc degeneration. International Journal of Clinical and Experimental Pathology, (2014); 7(2): 656–62.
- Ye Z, Wang C, Tang J, Zhou Y, Bai L, Liu Y, et al. Decreased interleukin-37 expression in Vogt-Koyanagi-Harada disease and upregulation following immunosuppressive treatment. Journal of Interferon & Cytokine Research, (2015); 35(4): 265–72.
- Isomaki P, Punnonen J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Annals of Medicine, (1997); 29(6): 499–507.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. TL – 36. Arthritis & Rheumatology, 1993; 36(6): 729–40.
- Kvien TK, Uhlig T, Odegard S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Annals of the New York Academy of Sciences, (2006); 1069: 212–22.
- Oliver JE, Silman AJ. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Research & Therapy,(2009); 9: 1–9.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0