Full Length Research Article
In vitro biochemical evaluation of methanol extract of Moringa oleifera pods on rat liver mitochondrial membrane permeability transition pore and lipid peroxidation
Cassandra Elohor Bezi, Adegoke Oluwaseyi Adetunji, Olaoluwa Temitope Talabi
Adv. life sci., vol. 5, no. 4, pp. 179-184, August 2018
*- Corresponding Author: Adegoke Oluwaseyi Adetunji (Email: olaoluwatemi@yahoo.com )
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Moringa oleifera is a well-known world herbal plant for its amazing medicinal and nutritional properties. The effect of methanol extract of M. oleifera can be useful in managing diseases associated with mitochondrial membrane permeability transition pore and lipid peroxidation.
Methods: Evaluation was done at varying concentrations of the methanol pods extract on mitochondrial membrane permeability transition pore opening (swelling) and Fe2+- H2O2-EDTA (Fenton reaction)-induced lipid peroxidation in vitro. Five male albino rats (weighed 120-250 g) were anaesthetized and sacrificed; the liver was excised and homogenized to obtain mitochondria pellets. This study analyzed the effect of varying concentrations of methanol pods extract of M. oleifera at 50, 150, and, 300 µg/ml respectively. The effects of M. oleifera varying concentration in vitro was determined using malondialdehyde reaction quantified at 532 nm in a UV- spectrophotometer as index for lipid peroxidation and spectrophotometric absorptions at 520 nm was observed as an index of mitochondrial membrane permeability pore respectively.
Results: Varying concentrations of methanol pods extract of M. oleifera at 50, 150, and 300 µg/ml in the presence and absence of triggering agent (Ca2+) inhibited opening of mitochondria membrane permeability transition pore while 0.25, 0.50 and 1.00 mg/ml inhibited lipid peroxidation induced mitochondria of the rat liver respectively in a concentration dependent mode.
Conclusion: The results suggest that methanol extract of M. oleifera pods at high concentrations (such as 300 µg/ml and 1.00 mg/ml respectively) may inhibit mitochondrial membrane permeability transition pore opening and lipid peroxidation.
Keywords: Moringa oleifera, mitochondria membrane permeability transition pore, lipid peroxidation
Plants are essential for human kind, animals, and environment in all viable ecosystems. They produce primary and secondary bioactive compounds which encompass a wide array of functions [1]. According to World Health Organization, medicinal plants are the most important source to derive a variety of novel herbal medicine. Around 80 % of persons from advanced and developing nations use conventional medicine, which has composites derived from medicinal plants. The Moringa oleifera tree is one of the world’s most useful herbs [2] and it is a well-known plant herb for its unique medicinal and nutritional properties [3]. The plant gives a rich exceptional mixture of zeatin, kaempferom, quercetin, β-sitosterol and caffeoylquinic acid [4,5] with substantial medicinal effects. Various parts of the plant serves as cardiac and circulatory stimulants, anti-tumour, anti-inflammatory, antioxidant, antipyretic, antiepileptic, antiulcer [6] and many other important medicinal properties.
The mitochondria are the powerhouse of the cells, which is useful for creating energy as adenosine triphosphate (ATP) and heat. They are also involved in the apoptosis-signaling pathway. One main functional attribute of mitochondria is the production of a huge transmembrane potential through the inner mitochondrial membrane. This process generates proton gradients which drives the production of ATP. The mitochondrial potential also powers the buildup of calcium into mitochondria. Calcium “overload” in the mitochondrial has been associated repeatedly in diverse disease mechanisms. The main process understood to facilitate calcium-induced mitochondrial damage is the mitochondrial membrane permeability transition (MMPT) pore [7], a huge conductance pathway in the inner mitochondria membrane that leads to ATP depletion, and necrotic cell death [8].
The MMPT pore may signify a channel for calcium to elude from excess mitochondria under biological states [9], it may play an essential function in form of some apoptotic cell death, as the opening of pores causes swelling of the mitochondrial and subsequent opening of the outer membrane with the release of cytochrome c [10]. Mitochondria are a main spring of free radicals, which an elevated concentration poses the threat of damage to cell membranes and DNA.
In these findings we explored the medicinal properties of M. oleifera effects on liver mitochondrial membrane permeability and lipid peroxidation to proffer additional information and knowledge to drug production for the treatment of lipid peroxidation and membrane permeability associated disorders.
Plant Collection, Processing and Extraction
Fresh pods of M. Oleifera were taken from a farmland at Irolu, Ikenne local government, Ogun state, Nigeria and were ascertained by Professor E.B. Esan of the Department of Basic sciences, Babcock University, Ilishan Remo, Ogun State. The pulverized test sample (100g) was dissolved in 70% methanol and shaken intermittently for 48 hours. Filtration of the test samples was done using the No.1 filter paper and the filtrate was concentrated in the water bath and preserved in the refrigerator pending further use.
Experimental Animals
Five male albino Wistar rats (weighing 120-250 g) were acquired from the department of physiology animal house, University of Ibadan, Ibadan, Nigeria. The rats were adapted for 15 days while been given water and rat chow ad libitum, and kept under accepted conditions of temperature and 12-hour dark/light cycle.
Experimental Design
A total of 5 male albino rats were used for the experimental study. The animals were anaesthetized with chloroform and subsequently sacrificed. The liver was excised and excess tissues trimmed, it was homogenized and centrifuged to obtain mitochondria pellets. This was further subjected to the swelling buffer assay test for the assessment of mitochondria membrane permeability transition (MMPT) pore using varying concentrations (50, 150 and 300µg/ml) of M. oleifera pods extract and the effect was investigated on the induction of lipid peroxidation in the rat liver mitochondria.
Preparation of Low Ionic Strength Rat Liver Mitochondria
Buffer C: 0.12 g of Hepes (Sigma Aldrich, USA) was dissipated in 70 ml of distilled water. 3.83 g mannitol (Sigma Aldrich, USA) was added and standardized to pH 7.4 with KOH (Sigma Aldrich, USA Products) and the whole solution made up to 100 ml. The buffer was then stored in the refrigerator at 4°C.
Buffer D: 0.12 g of Hepes (Sigma Aldrich, USA) was dissolved in 70 ml of distilled water. 3.83 g mannitol (Sigma Aldrich, USA) and Bovine Serum Albumin (Sigma Aldrich, USA) of 0.5 % were also added to the mixture and standardized to pH 7.4 with KOH (Sigma Aldrich, USA). The whole solution made up to 100 ml. The buffer solution was stored in the refrigerator at 4 °C.
Isolation of Liver Mitochondria from Rats
The liver mitochondria were isolated using the method by Johnson and Lardy (1967) combined with that of Schneider [11]. The excised and weighed rat liver was washed several times in buffer C until a clear wash was obtained and minced with a pair of scissors. The suspended liver homogenate in buffer C was implored into a refrigerated MSE centrifuge. The nuclear fraction and cellular debris were pelleted down by low speed centrifugation at 2300 x g for 5 minutes. The supernatant was re-centrifuged at the same speed and time to remove unbroken cells. The supernatant obtained was centrifuged at 13,000 x g for 10 minute to sediment the mitochondria. The brown mitochondrial pellet was washed by re-suspending in buffer D and centrifuged twice at 12,000 x g for 10 minutes. The obtained mitochondria were instantly suspended in an appropriate volume of ice cold Mannitol, Sucrose, Hepes pH 7.4-KOH (MSH) buffer then immediately dispensed in 1.5 ml eppendorf tubes in aliquot and kept on ice for immediate se. All assays were carried out in an ice-cold medium in order to preserve the integrity of the mitochondria.
Assessment of effects of methanol pods extracts M. oleifera on MMPT in Rat Liver Mitochondria
Swelling of the mitochondria was determined using the Lapidus and Sokolove method [12]. Intact mitochondria (0.4 mg/ml) were pre-incubated in 1.5 ml eppendorf tubes in the presence of varying concentrations of coartemether (50, 150 and 300 µl ) (which were all in iced conditions for 5 minutes), and in 0.8 µM rotenone for 3.5 minutes before adding 5 mM sodium succinate. Mitochondria were pre-incubated in 0.8 µM rotenone for 3 minutes, and Ca2+ was added, and Na succinate was added 30 seconds later. Swelling rate was quantified as a decrease in absorbance at 540 nm wavelength at a time interval of 30 seconds for 12 minutes with the use of JENWAY 6305 spectrophotometer. The sample blank was also taken into consideration every other condition including the mitochondrial buffer volume but excluding the mitochondrial therein.
Effects of Methanol Pods Extract M. oleifera on Lipid Peroxidation
The induction of lipid peroxidation was done using Fe2+-EDTA system in rat liver mitochondria in the presence and absence of methanol pods extracts of M. oleifera of varying concentrations to produce thiobarbituric acid reacting constituents (TBARS) which contained a mixture of 0.1 ml of mitochondria in MSH buffer pH 7.4, 0.1 ml of FeSO4, 0.1 ml of EDTA, 0.1 ml of H2O2, and 0.5 ml of various concentrations (0.25, 0.5 and 1.0 mg/ml) of methanol pods extract of M. oleifera to a concluding volume of 1 ml. The reaction mixture was incubated for 1 hour at 37 ºC. After incubating 0.5 ml of the reaction mixture was treated with 0.2 ml of SDS, 1.5 ml of TBA and 1.5 ml of acetic acid respectively. The TBARS thus formed was determined by the method of Ohkawae et al. [13]. The total volume was measured up to 4 ml by distilled water and kept in a water bath at 95-100ºC for 1 hour. Two milliliters of the reaction mixture was then mixed with 3 ml of n-butanol (BDH Chemicals Ltd, England), vortexed and centrifuges at 3000 x g for 10 min, the organic layer was removed and its absorbance was measured at 532 nm in a UV-spectrophotometer. Tubes without Fenton’s reaction mixture or M. oleifera pod extract but with rat mitochondria were included as blank, while those without M. oleifera pods extract alone were taken as control. The mean absorbance of the M. oleifera pods extract treated tubes was compared with that of Fenton’s reaction alone to determine the lipid peroxidation. The experiments were done in triplicates.Data analysis was carried out using Microsoft Office Excel, Standard version 2007 to determine percentage inhibition.
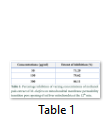
Data analysis showed an intact mitochondrial membrane in figure 1 when the absorbance of the mitochondria suspension was read with no calcium chloride (triggering agent). In the presence of calcium chloride there was decrease in absorbance of the mitochondrial suspension at 540 nm, an indication mitochondrial swelling, while the spermine inhibited mitochondrial swelling (figure 1). The rate of mitochondria swelling decreases with increasing concentrations (50, 150 and 300 µg/ml) of methanol pods extract of M. oleifera (figure 2). Minimal (71.29%) and maximal (86.11%) inhibitions of mitochondria swelling were at 50 and 300 µg/ml of methanol pods extract of M. oleifera respectively (table 1). Furthermore, spermine inhibited MMPT at 50, 150 and 300 µg/ml concentrations of methanol pods extract of M. oleifera (figure 3).
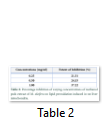
The varying concentrations (0.25, 0.50 and 1.00 mg/ml) of methanol pods extract of M. oleifera stimulated an increase in the percentage inhibition of lipid peroxidation in a concentration dependent manner with minimal (21.31%) and maximal (37.22%) inhibitions at 0.25 and 1.0 mg/ml respectively (table 2).
Tables & Figures
The varying concentrations of methanol pods extracts of M. oleifera inhibited the opening of the MMPT pore in a concentration dependent manner. Thus, M. oleifera pods might be able to improve mitochondrial function by reducing the rate of occurrence of mitochondrial related diseases [14]. Oxidative damage occurs and pileup in the mitochondria when reactive oxygen species (ROS) such as the superoxide radical are not converted to water (H2O) fast enough by superoxide dismutases and peroxidases. Radical species, such as hydroxyl ions (OH−) and hydrogen peroxide (H2O2) may be present in high concentrations, posing a risk of lipid peroxidation. A major significance of uncontrolled oxidative stress (imbalance between the prooxidant and antioxidant levels in favor of prooxidants) is cells, tissues, and organs injury caused by oxidative damage. It has long been acknowledged that high levels of free radicals or ROS can perpetrate direct damage to lipids. An equilibrum between the production of oxidants and the scavenging of those oxidants by antioxidants establishes the extent of lipid peroxidation.
In this study, the various concentrations (0.25, 0.50 and 1.00 mg/ml) of M. oleifera pods used protected against Fe2+- H2O2-EDTA (Fenton reaction)-induced lipid peroxidation in rat liver mitochondria homogenate in a concentration dependent manner at 21.31, 24.15 and 37.22 percentage inhibition respectively. The methanol pods extract of M. oleifera may have performed protection either by neutralizing the H2O2 or by scavenging the superoxide (.OH) generated from the Fenton’s reaction. This coincide with similar report by Kumar and Pari (2003) [15], that M. oleifera pods possess anti-oxidant properties which may help in maintaining health when continuously taken as part of dietary foods, spices or drugs.
The protective property of methanol extract of M. oleifera pods could be due to the fact that M. oleifera pods contain essential bioactive compounds] isothiocyanates, like glucosinolates, flavonoids, and thiocarbamates [16]. These compounds regenerate membrane-bound antioxidants, satiates ROS, and chelate metal ions. This finding is consistent with earlier studies, which demonstrated the antioxidant activity of M. oleifera pods extracts [14,15].
The methanol extract of M. oleifera pods could be an inhibitor of MMPT pore opening and an inhibitor of lipid peroxidation by reducing the risk of diseases associated with mitochondria dysfunction and oxidative stress when regularly taken as constituent of herbs, dugs or dietary foods. The alkaloids, flavonoid and phenol components of the M. oleifera can be credited for these observed effects. Thus, the isolation and characterization of specific compounds involved in the observed effect by methanol extract of M. oleifera pods will give more credence to these findings.
Conflict of interest
Authors declare that there is no conflict of interest for publishing this study.
- Croteau R, Kutchan TM, Lewis NG. Natural products (secondary metabolites). In: B. Buchanan, W. Gruissem, R. Jones, (Eds.), Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiology, (2000); 1250-1318.
- Devendra BN, Shrinivas N, Prasad TV, Swarna L. Antimicrobial activity of Moringa oleifera leaf extract, against selected bacterial and fungal strains. International Journal of Pharma Bioscience and Technology, (2011); 2(3): 13-18.
- Patil SD, Jane R. Antimicrobial activity of Moringa oleifera and its synergism with Cleome viscose. International Journal of Life Sciences, (2013); 1(3):182-189.
- Sharma V, Paliwal R. Isolation and characterization of saponins from Moringa oleifera (moringaeceae) pods. International Journal of Pharmacy and Pharmaceutical Sciences, (2013); 5(1), 179-183.
- Paliwal R, Sharma V, Pracheta S, Yadav S, Sharma S. Anti-nephrotoxic effect of administration of Moringa oleifera Lam in amelioration of DMBA-induced renal carcinogenesis in Swiss albino mice. Biology and Medicine, (2011); 3(2): 27-35.
- Pal SK, Mukherjee PK, Saha BP. Studies on the antiulcer activity of M. oleifera leaf extract on gastric ulcer models in rats. Phytotherapy Research, (1995); 9: 463-465.
- Crompton M, Barksby E, Johnson N, Capano M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie, (2002); 84: 143–152.
- Duchen MR, Mitochondria in health and disease: perspectives on a new mitochondrial biology. Molecular Aspects of Medicine, (2004); 25(4): 365–451.
- Petronilli V, Penzo D, Scorrano L, Bernardi P, Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death: correlation with the duration of pore openings in situ. Journal of Biological Chemistry, (2001); 276: 12030–12034.
- Petit PX, Goubern M, Diolez P, Susin SA, Zamzami N, Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Letters, (1998); 426:111 –116.
- Lardy HA, Wellman H. The catalytic effect of 2,4 dinitrophenol on adenosine triphosphate hydrolysis by cell particles and soluble enzymes. Journal of Biological Chemistry, (1953); 201(1): 357-370.
- Lapidus RG, Sokolove P M. Spermine inhibition of permeability transition of isolated rat liver mitochondria: an investigation mechanism. Archive of Biochemistry and Biophysics, (1993); 306(1): 246-253.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Annual Review of Biochemistry, (1979); 95: 351-8.
- Arabshahi DS, Devi V, Urooj A. Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability. Food Chemistry, (2007); 100: 1100–1105.
- Kumar A, Pari L. Antioxidant action of Moringa oleifera Lam (drumstick) against antitubercular drugs induced lipid peroxidation in rats. Journal of Medicinal Food, (2003); 6: 255–259.
- Bharali R, Tabassum J, Azad MR. Chemomodulatory effect of Moringa oleifera, Lam, on hepatic carcinogen metabolising enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pacific Journal of Cancer Prevention, (2003); 4: 131–139.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0