Biochemical basis of resistance in rice against Bacterial leaf blight disease caused by Xanthomonas oryzae pv. oryzae
Junaid Ahmed Khan1, Sehar Afroz2, Hafiz M. Imran Arshad3, Nighat Sarwar3, Hafiza Sidra Anwar3, Kamran Saleem3*, M Masood Babar3, Farhat Fatima Jamil3
Adv. life sci., vol. 1, no. 3, pp. 181-190, May 2014
*-Corresponding Author: Kamran Saleem (Kammmran@hotmail.com)
Author Affiliations[Date Received: 09/05/2014; Date Revised: 19/05/2014; Date Published Online: 25/05/2014]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Bacterial Leaf Blight (BLB) is the most devastating disease of rice. This disease can reduce grain production by 20-50% and yield by 25%. The disease is widespread in Asia, United State, Latin America and Australia. In Pakistan, it is reported from all rice growing areas and it is increasing its area year by year. All famous Pakistan’s Basmati varieties are susceptible to BLB disease. The most economical way of controlling disease is to produce resistant varieties. Many different compounds are involved in resistance mechanism in host including phenolic compounds.
Methods: Plants inoculated with Xanthomonas oryzae pv. oryzae isolates i.e. 78.3 and 2.2 caused disease on all varieties were selected for investigation of phenolic compound. Randomized complete block design was used in field for inoculation and leaf collections for phenolic compounds determination. Data was phenolic compounds were analyzed through factorial ANOVA.
Results: The response of four varieties was different when inoculated with BLB isolate 2.2. Basmati 2000 variety of rice produced maximum amount of phenolic compounds after one week of inoculation of bacterial isolate 2.2 while the minimum total phenols were found in basmati 385 at 0 hrs. of inoculation (before inoculation). Xoo isolate 78.3 produced maximum phenolic compounds after 2 weeks in Basmati 2000.
Conclusions: Biochemical resistance due to high phenolic contents in Basmati-385 and Basmati-2000 is suggested. High phenol production may be due to loss of virulence in bacterial isolates. Both 78.3 and 2.2 Xoo isolates are significantly different in producing total phenols in all the varieties tested.
Keywords: Phenol, Basmati rice, Systemic resistance, Bacterial Leaf Blight
Introduction
Bacterial leaf blight (BLB) disease, caused by Xanthomonas oryzae pv. oryzae (Xoo), is one of the most serious diseases in most rice growing countries due to its high epidemic potential and destructiveness to high-yielding cultivars in both temperate and tropical regions [1]. Bacterial leaf blight was first noticed by the farmers in Japanin 1884-85 [2]. In Pakistan, BLB was first reported by Mew and Majid in 1977 and its incidence was reported as 10-15, 15-20, 20-25% in Sindh, Punjab and KPK respectively [3]. BLB was found in all northern districts including Muridke, Narang and adjoining areas [4].
BLB occurs at all the growth stages of rice and is manifested by either leaf blight or “kresek” symptoms. If plant ever produces panicles, it results in sterile immature grains, which are easily broken during milling. Yield reduction has been recorded about 10-12% in case of mild infection [5] and in case of severe infection reduction in yield has been recorded 50% [6]. When there is heavy infection, no grain formation takes place. The causal organism consists of straight rods, with a single polar flagellum, occurs singularly or in pairs or sometimes in chains and is gram negative [7]. The bacterium enters the plants through natural openings or wounds where it survives and multiplies in plant's vascular system, producing typical leaf blight symptoms.
Defense strategies of plants against pathogens are several, including the production of antifungal chemicals, which are either pre-formed (i.e. already present in plant tissue in different amounts) or induced following infection (e.g. de novo synthesized phytoalexins). Many of the phytoalexins or pre-formed chemicals belong to the phenolic group [8]. Phenolic compounds are secondary metabolites which are synthesized in plants. They play a role in plant defense against pathogens. Accumulation of these secondary metabolites in plants may play a major role in plant defense response. Their activity is related to antimicrobial properties, involvement in cell wall reinforcement, modulation and induction of plant responses [9]. Generally, when a plant is infected, its phenolic content increases, as a consequence of a defense reaction to infection [10].
All famous Pakistan’s Basmati varieties are susceptible to BLB disease [11]. The present work was designed to study the role of phenols involve in resistance against Xanthomonas oryzae pv. oryzae in four rice basmati varieties by the comparison in total phenols production before and after inoculation.
Methods
Collection of diseased material: The rice leaves affected with bacterial leaf blight were collected by plant protection division, Nuclear Institute for Agriculture and Biology (NIAB). The diseased plants with BLB were recognized with appearance of yellow to white water-soaked stripes at the margins of infected leaves. Some heavily infected plants were seen with the characteristic yellowish lesions with wavy edges mainly in the upper part of the effected leaves.
Isolation and purification of pathogen: Infected leaves were cut into small pieces, washed twice with autoclaved distilled water and left in autoclaved distilled water for 20 minutes [4]. Leaf pieces were then placed and bacterial ooze in water was streaked on Nutrient Agar (NA) medium (Nutrient broth 2.5%, Agar 2%) plates and left in incubator at 30°C overnight for the isolation of Xanthomonas oryzae pv. oryzae. After one night, yellow colonies were picked and purified on fresh NA plates. Identification and characterization of pathogen was done by morphological examination and Gram staining method and biochemical tests (Indole test, Voge-Proskauer’s test (MR-VP), Methyle Red test and Citrate test).
Estimation of total phenols in basmati varieties of rice: Four varieties of basmati rice (Basmati-370, Basmati-385, Basmati-2000 and Basmati-super) were sown in glass house. 60 days old rice plant varieties were inoculated with bacterial culture prepared in nutrient agar medium for 48 hrs. on water shaker at 35 °C. Extraction and determination of total phenols were made according to Sarwar et al, 2001 [12]. Plants inoculated with isolate 78.3 and 2.2 caused disease on all varieties were selected for investigation of phenolic compounds. Samples of leaves were collected from healthy and diseased plants of all four of basmati on zero hour, 24 hours, one week and two weeks after inoculation. On zero hour sampling leaves were inoculated (without bacterial inoculation). Each plant sample was weighed 1 g and cut into small pieces and put into methanol and boiled for 5 minutes until the green color came out. Methanol was decanted and plant tissues were homogenized in tissue homogenizer polytron. The samples were again boiled for 5 minutes and then filtered it through whatman No.1 filter paper and residue were washed with 80% acidified (0.1 % HCl conc.) methanol. Methanol collected from step 1 and filtrates were pooled together and then methanol was evaporated by using Rota evaporator at 45o C and collected the aqueous layer. Aqueous portion of extract was washed with n-hexane in separating funnel three times until no green color in aqueous layer was left. Final volume of extract was made as 2ml for zero hour samples and 4ml for 48 hours, one week and 2 weeks, with distilled water which was used for determination of total phenols. The concentration of phenolic compounds in the above water extracts was determined by using the Folin Ciocalteau reagent according to the modified method [13]. The 0.2ml of extract were taken in test tubes and added 4ml of 4% Na2CO3 solution was added, then 0.2 ml of Folin Ciocalteus reagent was added with constant shaking on vortex mixer. Absorption was measured in double beam spectrophotometer at 750 nm after 30 minutes.
Results
Morphological and Biochemical characterization of Xoo isolates:
Colonies of Xanthomonas oryzae pv. oryzae were cultured on Nutrient Agar plates. Colonies were yellow, circular, translucent, smooth, convex, opaque and even in surface and margin. For the identification of Xanthomanas oryzae pv. oryzae microscopic studies were carried out by Gram staining method. Isolates were found Gram-negative and rods were cultured on NA slants and stored in refrigerator at -4°C.Confirmation of pathogen was also carried out by biochemical characterization of the isolated bacteria. Four Biochemical tests (Indole, Voges-Proskauer’s (MR-VP), Methyl Red and Citrate tests) were performed for the biochemical characterization of the Xoo isolates. Both isolates showed negative reaction to Indole, and VP while positive to Citrate utilization. Isolate 2.2 showed positive response to Methyl red test while 78.3 showed negative result (Table 1).
Estimation of total phenols concentration:
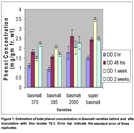
The results of total phenols found in four basmati varieties before and after inoculation with Xoo isolate no. 78.3 and 2.2. Basmati-Super variety of rice produced maximum amount of phenolic compounds after one week of inoculation of bacterial isolate 78.3 while the minimum total phenols were found in basmati 385 at 0 hrs. of inoculation (Fig. 1). After 48 hours of inoculation, Basmati 2000 contained maximum phenolic compounds followed by Basmati-super while after one week of inoculation no significant difference in phenol concentrations was observed between Basmati 385 and Basmati 2000. Overall different trends in phenol concentration at different timings in different varieties was observed which may be due to different resistant genes and biochemical pathway of resistance.
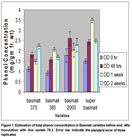
The response of four varieties was different when inoculated with BLB isolate 2.2. Basmati 2000 variety of rice produced maximum amount of phenolic compounds after one week of inoculation of bacterial isolate 2.2 while the minimum total phenols were found in basmati 385 at 0 hrs. of inoculation (Fig. 2).
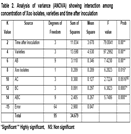
Both Basmati 2000 and Basmati super contained maximum phenolic compounds after 1 week of inoculation. In Basmati 370, an increasing trend in phenol concentration was observed with respect to time after inoculation while for rest of three varieties have different trend of phenols accumulation. Analysis of variance indicate that both Xoo isolates 78.3 and 2.2 differ significantly in induction of phenols production in four rice varieties at different timings (Table 2). Significant interaction was observed between four rice varieties and times after inoculation. Behavior of four rice varieties was different at different time for phenol accumulation. Four varieties interact with Xoo isolates with respect to phenol accumulation and behavior of four varieties was significantly different. Significant difference in phenol accumulations was observed in four basmati varieties at 0hours to 2 weeks. Inoculation with different Xoo isolates have significant effect on phenol accumulation but matter of time of phenol accumulation and varieties were of big concern. Although Basmati super accumulate phenolic compounds, significantly different against both isolates of Xoo but that may be due to innate resistance of Basmati super against Bacterial leaf blight or both isolates have different level of virulence. If a variety accumulates phenolic compounds immediately after inoculation i.e. 48 hours to 1 week, although higher concentrations of phenols are phytotoxic” that will lead to hypersensitive response and whole resistance cascade of rice plant become activated. Decrease in phenolic compounds accumulation in later stages may be because of two reason. One may be due to phytotoxic effect of phenolics and second may be the successful infection of Xoo isolate which make variety susceptible. Similar trend was observed in rest of three varieties (Table 3).
Discussion
Defense strategies of plants against pathogens are several, including the production of antifungal chemicals, which are either pre-formed (i.e. already present in plant tissue in different amounts) or induced following infection (e.g. de novo synthesized phytoalexins). In both groups of chemicals, those synthesized in secondary metabolism are especially interesting. Many of the phytoalexins or pre-formed chemicals belong to the phenolic group [8]. Phenolic compounds are secondary metabolites which are synthesized in plants. They not only play a role in plant defense against pathogens but also possess biological properties such as: antioxidant, anti-apoptosis, anti-aging, anti-carcinogen, anti-inflammation, anti-arthrosclerosis, cardiovascular protection, improvement of the endothelial function, as well as inhibition of angiogenesis and cell proliferation activity. Most of these biological actions have been attributed to their intrinsic reducing capabilities. In the current study phenolic compounds produced in four different rice varieties against two Xoo isolates were analyzed.
Confirmation of pathogen is a prerequisite and essential part of the studies. For this purpose morphological, microscopic and biochemical characterization of the isolated bacteria was recorded. Our observations were very much similar with the already reported characteristics of Xanthomonas oryzae pv. oryzae [14]. Four Biochemical tests (Indole, Voges-Proskauer’s (MR-VP), Methyl Red and Citrate tests) were performed for the biochemical characterization of the Xoo isolates. Both isolates showed negative reaction to Indole, and VP while positive to Citrate utilization. Isolate 2.2 showed positive response to Methyl red test while 78.3 showed negative result. Methyl red test detects the production of acid during fermentation of glucose. Red color is positive and yellow shows that it is a negative bacteria, so it very helpful in bacterial identification. This assay determines if an organism metabolizing pyruvic acid utilizes mixed acid pathway and produces acid end products that are detected by the indicator methyl red. Positive MR is indicated if the methyl red reagent remains red. Negative result is indicated if the reagent turns yellow-orange. A positive result is indicated by the presence of a red color that develops within 15 min. No color change indicates negative VP [15]. Xanthomonas spp. exhibits negative results to Indole, Methyl Red and Voges Proskauer’s tests. Our results (Negative to all three tests) are in agreement with the already reported characteristics of Xanthomonas spp [16].
Phenol concentrations were found significantly different in different treatments. Maximum amount was found in third treatment i.e. after one week of inoculation while minimum concentration was observed at first treatment that is before inoculation. Significant differences were found among the behavior of varieties in production of phenolic compound against the infection of Xoo isolates. Basmati Super variety of rice produced maximum amount of phenolic compounds after one week of inoculation of bacterial isolate 78.3. The bacterial isolates 78.3 and 2.2 are also significantly different in inducing phenol production. Bacterial isolate 78.3 induced higher phenol accumulation in super variety of rice after one week of inoculation. Interaction of treatment and variety also has pronounced effect. Maximum phenolic compounds were observed after one week in Basmati super variety of rice. Combination of treatment with bacterial isolates has no effect on phenol accumulation while different varieties show different response against different bacterial isolates.
Our results show that phenol concentration gradually increases after inoculation but in susceptible variety accumulation is very slow. As bacteria cause successful infection, phenol concentration starts to reduce. Rice varieties Basmati-385 and Basmati-2000 are susceptible against both bacterial isolates although phenolic compounds start to accumulate but reduce after one week which may be due to successful infection or deterioration of phenolic compounds within the plant. In basmati super and basmati 370 varieties of rice phenols accumulate at faster rate and in high concentration but in second week of inoculation phenolic compounds in plants start to reduce which may be due to phytotoxic effect of the pathogen or higher phenolic compounds/concentration in pathogen as compared to plants. Similar results were previously reported [17]. He found that phenolic contents in rice leaves against Xoo increased significantly one day after inoculation and the maximum accumulation of phenols was observed two days after inoculation. Phenolic compounds accumulate in higher concentration and at faster rate in systemic acquired resistance after induction through chemical signal or through pathogen.
In conclusion, rice varieties Basmati-385 and Basmati-2000 are susceptible while Basmati-super and Basmati-370 are moderately susceptible. Some sort of resistance due to high phenolic contents can also be suggested which may be due to loss of virulence in bacterial isolate 78.3 because both Xoo isolates are significantly different in producing total phenols in all the varieties tested. Biochemical resistance is very complex mechanism so in addition to phenolic compounds, accumulation of peroxidase, catalase and other pathogenesis related proteins should also brought under consideration. The finding of this study will be helpful for the rice breeders to determine those genes which trigger phenol production or genes involve in phytoalexin production. Management of Xoo can be achieved through spray of those chemical compounds which trigger phenol production.
- Subramoni S, Sonti RV. Growth deficiency of a Xanthomonas oryzae pv. oryzae fur mutant in rice leaves is rescued by ascorbic acid supplementation. Molecular plant-microbe interactions, (2005); 18(7): 644-651.
- Ezuka A, Kaku H. A historical review of bacterial blight of rice. Bulletin of the National Institute of Agrobiological Resources, (2000); (15): 1-207.
- Mew T, Majid A. Bacterial blight of rice in Pakistan. IRRN, (1977); 2(1): 5.
- Khan J, Jamil F, Gill M. Screening of rice varieties/lines against bakanae and bacterial leaf blight (BLB). Pakistan Journal of Phytopathology, (2000); 12(1): 6-11.
- Akhtar MA, Zakria M, Abbasi FM. Inoculum build up of bacterial blight of rice in rice-wheat cropping area of Punjab in relation to zero tillage. Asian J Plant Sci, (2003); 2548-550.
- Mew T, Alvarez A, Leach J, Swings J. Focus on bacterial blight of rice. Plant disease, (1993); 77(1): 5-12.
- Ali A, Khan MH, Bano R, Rashid H, Raja NI, et al. Screening of Pakistani rice (Oryzae sativa) cultivars against Xanthomonas oryza pv oryzae. Pakistan Journal of Botany, (2009); 41(5): 2595-2604.
- Grayer RJ, Kokubun T. Plant–fungal interactions: the search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry, (2001); 56(3): 253-263.
- Puupponen‐Pimiä R, Nohynek L, Meier C, Kähkönen M, Heinonen M, et al. Antimicrobial properties of phenolic compounds from berries. Journal of applied microbiology, (2001); 90(4): 494-507.
- Gessler C, Patocchi A, Sansavini S, Tartarini S, Gianfranceschi L. Venturia inaequalis resistance in apple. Critical Reviews in Plant Sciences, (2006); 25(6): 473-503.
- Khan JA, Arshad HMI, Jamil FF, Hasnain S. Evaluation of rice genotypes against bacterial leaf blight (BLB) disease. Pak J Phytopathol, (2009); 21(1): 26-30.
- Sarwar N, Sarwar M, Jamil F. Characterization of Ascochyta rabiei isolates using random amplified polymorphic DNA (RAPD) technique. Pakistan Journal of Phytopathology, (2000); 12(1): 18-25.
- Bray H, Thorpe W. Analysis of phenolic compounds of interest in metabolism. Methods of Biochemical Analysis, Volume 1, (1954); 27-52.
- Agarwal P, Mortensen CN, Mathur S. Seed-borne diseases and seed health testing of rice. Phytopathological papers, (1989); (30).
- Gilligan PH, Lum G, Vandamme P, Whittier S. Burkholderia, Stenotrophomonas, Ralstonia, Brevundimonas, Comamonas, Delftia, Pandoraea, and Acidovorax. Manual of clinical microbiology, (2003); 729-748.
- Ogunjobi A, Fagade O, Dixon A. Physiological studies on Xanthomonas axonopodis pv. manihotis (Xam) strains isolated in Nigeria. Eur J Biol Sci, (2010); 284-90.
- Velazhahan R, Jayaraj J, Khabbaz S, Kumar RS, Muthukrishnan S. Xanthomonas oryzae pv. oryzae infection triggers accumulation of phenolics, defense-related enzymes and thaumatin-like proteins in rice leaves. Archives of Phytopathology and Plant Protection, (2006); 39(5): 329-339.