Full Length Research Article
Impact of IL28B gene variants (rs12979860) in peg-IFN therapy against Chronic Hepatitis B Pakistani patients
Irfan Kalam, Sajjad Ullah, Qaisar Ali, Arshad Jamal, Ahmad Bilal Waqar*
Adv. life sci., vol. 6, no. 1, pp. 11-18, November 2018
*- Corresponding Author: Ahmad Bilal Waqar (Email: drabwaqar@yahoo.com)
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Genome wide association studies elucidate that IL28B rs12979860 genetic polymorphism has a substantial role as a pretreatment predictor during PEG-IFN therapy in Hepatitis C virus (HCV) infection, however its role in chronic hepatitis B (CHB) is ambiguous.
Methods: In this study, we have investigated the role of IL28B variant rs12979860 for chronic hepatitis B (CHB) infection. 200 CHB patients were treated for 24 weeks, then we carried out our study on these patients. Moreover, all patients were investigated for IL28B rs12979860 genotypes CC, CT, TT through RT-PCR. Based on our preliminary results we categorized these patients into two groups i.e. Responder ¢R (n = 104) and Non-responder¢NR (n = 96).
Results: The proportion of IL28B CC, CT, TT genotypes were in both responder and non-responder groups as (72.1%, 25.0%, 2.9% vs 53.1%, 40.6%, 6.2% respectively; P = 0.02). In this study CC was the most frequent genotype, which has significant outcome in PEG-IFN therapy in both R and NR groups (72.1% vs 53.1%) (P = 0.03), while CT and TT were found as (25.0% vs 40.6%) (P = 0.1, 2.09% vs 6.2%) (P = 0.3) respectively.
Conclusion: Current study clearly demonstrates that IL28B rs12979860 polymorphism is a pretreatment predictor during PEG-IFN therapy in CHB infection, and patients with favorable genotype of rs12979860 (CC) may clear the virus than the non-favorable genotypes (CT, TT) in Pakistani population.
Keywords: IL28B genetic polymorphism, PEG-IFN, Chronic Hepatitis B, Chronic Hepatitis C
Hepatitis B virus (HBV) is worth considering and challenging global health issue in general and Asia in particular [1-4]. It has badly effected 02 billion humans including 400 million chronically, and claiming the lives of 620,000 annually. Likewise, it is prevalent in our country (Pakistan) and higher proportion of the population is affected (09 million), while it is scaling up with each passing day [2,4-9]. Hepatitis B surface antigen (HBsAg) persist for more than six months in the serum is considered chronic hepatitis B (CHB) infection [10,11]. CHB is the leading cause of morbidity and mortality in developing countries. It is responsible for 53% of hepatocellular carcinoma (HCC) and 30% of cirrhosis cases [12]. If CHB infection persists up to five years it leads to cirrhosis, causing death, yet 15-40% cases can be compensated and the rest persist in chronic stage. [13,14]. CHB may progress from cirrhosis to advance stages HCC and Hepatic Decompensation. Approximately 15-20% of CHB patients can progress to these serious stages [15-19]. The widespread mortality rate is the cause of the close association with the complex nature of the virus and host immune system [20,21].
Three cytokines (IL28A, IL28B and IL29) have been found in the study of GWAS on chromosome no. 19 (q13), the second mentioned cytokine (IL28B) is coded for type ш IFN family and they are IFN-k1, IFN-k2 and IFN-k3 which play vital role in generating antiviral response by activation of JAK-STAT and MAPK pathways [22-24]. The 1st ever study about IL28B was reported in 2010, showing IL28B is linked with spontaneous clearance in HCV. Whereas, in 2012, the 1st influential report about IL28B also showed sustained virological response (SVR) in CHB. Furthermore Sonneveld reported that IL28B polymorphism has significant association in HBeAg seroconversion and also increase loss of HBsAg in response to peg IFN [25-29]. An individual who possesses CC genotypes can give better response to the treatment and clear the virus rapidly [30]. Li ¢s study containing two groups (healthy control and self-limiting the infection) concluded that CHB patients had low level of IL28B in their serum, and also stated that patients with CC genotypes had higher level of IL28B in their serum than the non-CC [31].
To prevent the patients from advance stages and to stop the progression of liver disease is the ultimate aim of the therapy, based on two group of antiviral drugs nucleotide analogues (NAs) and IFN. The former mentioned having the following drugs (Lamivudine, Entecavir, Telbivudine, and two NAs prodrug Tenofovir Disoproxil Fumarate, and Adefovir Disoproxil) while the later have (conventional IFN and PEGylated IFN) [32-35]. Conventional IFN is known as the 1st registered drug for the treatment of CHB but has proven ineffective due to weak response in patients with normal levels of alanine amino transferase (ALT), adverse effects and complexity in drug administration. Peg-IFN mediated treatment showed remedial steps which eventually leads to HBeAg seroconversion and the rendered patients with low risk of cirrhosis and HCC [36-40]. In the result of phase 3 trials, peg-IFN has turned the axis of interest of the researchers and the trials demonstrated that peg-IFN gives desired results as compared to conventional IFN. It is further revealed that unlike NAs, peg IFN showing no antiviral resistance because it is given to the patient for definite time. Conventional IFN is prescribed thrice and peg-IFN one dose per week. Peg-IFN suppresses virus significantly, eradicates HBsAg satisfactorily and normalizes ALT levels [41-44].
This study is to understand the role of IL28B gene variants in the treatment of peg-IFN in CHB infected individuals in Pakistani population.
Patients
In the present study 355 CHB patients of Pakistani origin were included (Figure 1), 155 were dropped due to adverse effects of the given therapy, dose negligence or missing clinical history. Whereas 200 cases were followed on and were given peg-IFN with proper dose of 180 (million international units per week IM or subcutaneously), while the physicians prescribed the dose in some patients according to their health condition. The selected patients have a complete follow up history and they also have completed their 06 month regiment of peg-IFN. In these selected cases, 128 were males and 72 were females. These patients are categorized into two groups as, Responders (R) and Non-responders (NR). The inclusion criteria for the study are, patients having chronic HBV infection and have successfully completed their 06 months of peg-IFN-Alfa therapy. The patients tested positive for other hepatic infections such as hepatitis A, C, D and E, some other viral disease or have some other severe medical condition were excluded from the study along with pregnant and women in lactation. The study protocols were approved by the ethical committee of Imperial College of Business Studies – Faculty of Allied and Health Sciences – Lahore. Inform consents were received from all the participants of the study.
Laboratory work-up
For antigen antibody detection serum was used and for IL28B genotyping EDTA whole blood was processed. Serum was used to investigate co-infections like antibody to HDV, HIV, and HCV by a simple technique called enzyme linked immunosorbent assay (ELISA). HBV DNA was extracted from EDTA blood plasma by using FavorPrep TM viral nucleic acid extraction kit by strictly following the instruction on the kit. HBV quantification was performed on HBV Real Time Quant (Sacace Biotechnologies, Como, Italy) on M.J mini personal thermo cycler Real time PCR System (BIO-RAD) use according to the given guidelines.
IL28B genotyping
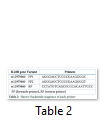
EDTA whole blood samples were used of all the patients for the extraction of genomic DNA by using non-enzymatic salting out protocol. Real time PCR was used for the Genotyping of IL28B rs12979860. Proper environment was provided to StepOne Biosystem to perform amplification. Specific primers i.e. Forward primer-1 sequence is 5- AGG GAG CTC CCC GAA GGC GC-3 (Forward primer 2 sequence is 5¢- AGG GAG CTC CCC GAA GGC GT-3 (and Reverse primer sequence is 5¢-CCT ATG TCA GCG CCC ACA ATT CCC A-3¢ were used table 2. These primers were specifically designed for 190 bp and used for SNP rs12979860 genotypes CC, CT, and TT. The reaction mixture contained 120-480 ng (2.5 µl) of DNA, 01 µl of each primers, 2.5 µl MgCl2, 10X-buffers 2.5 µl, dNTPs 2.5µl, 0.5 µl of Taq DNA polymerase and nuclease free H2O were 12.5ml the total volume of the master mix in each reaction was 25 ml. Thermocycler temperature was set as; initial denaturation 95°C for 05 minutes, annealing 68°C for 30 seconds and extension 72°C for 30 seconds. These all steps were programmed in real time PCR (StepOne Applied Biosystem).
Statistical analysis
SPSS statistic software package version 20.0 was used to perform data analysis. Data were analyzed as; qualitative variables show frequencies and continuous variables were expressed as median and mean. Independent T-Test was carried out to find the differences in frequencies of continuous variables. For qualitative variables Pearson chi-square test was used. Hardy Weinberg equilibrium is used to find out the significance of IL28B polymorphism.
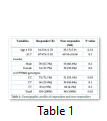
HBV genotype A, B, C, and D patients were carried out in this study, IL28B genetic polymorphism (rs12979860) was investigated in all patients categorized into two Groups i.e. Responders (R) and Non-Responders (NR). Patients whose viral load were less than 2000 copies IU/ml were regarded (R category) n = 104, and those patients with more than 2000 copies IU/ml after completion of treatment were put into (NR) category n = 96 (Figure 1). Hardy Weinberg equilibrium was applied to both categories (R=0.09, NR=0.14). The mean ± SD age in the R category was (Mean ± SD, 34.53±11.76) and in NR category (Mean ± SD, 33.17±07.91) (p = 0.34). The mean ± SD of ALT levels in the R category were (Mean ± SD, 67.97±11.02) and in NR category (Mean ± SD, 69.53 ± 10.06) (p = 0.29) (Table 1).
Out of 200 cases, males were n = 128 (64.0%) (p value=0.28) and in the R category n = 70 (35.0%), and in N.R category (n = 58 (29.0%), (p = 0.28) (Figure 2). 72 females were investigated in a study, R group (n=34) (32.7%) and in NR (n=38) (39.6%), (p = 0.63) in both R and NR categories. Gender wise (male and female) (p = 0.31) which is insignificant in response to R and NR groups.
Figure 4 shows the electrophoresis pattern of IL28B (rs12979860) genotypes. In IL28B polymorphism (rs12979860) CC genotypes were more frequently in our region than non-CC genotypes (Figure 3) i.e. (CC = 63.0%, CT = 32.5%, TT = 4.5%). CC genotypes were abundant in both R (n = 75) (72.1%) and NR (n = 51) (53.1%), (p = 0.03) it shows a significant association between R and NR groups. Patients with CT genotype were (n = 26) (25.0%) in R category, and in NR (n = 39) (40.0%) (p = 0.1) which reflects insignificant association between R and NR- categories. TT genotype was the least frequent genotype in both the categories (R (n = 03) (2.9%), NR (n = 06) (6.2%) (p = 0.3).
The IL28B polymorphism (CC CT TT) demonstrated significant association in the treatment of peg-IFN among the R and NR groups (p = 0.02) (Figure 3). This study demonstrates that CC genotype was the most frequent genotype in our region and it has a better response to peg-IFN therapy in both (R and NR) categories compare to CT and TT genotypes. The present study did not find any significant variation between gender and age wise distribution in both R and NR groups.
Tables & Figures
Despite a lot of work in the last few decades to understand the nature of the HBV, still many aspects are unclear. Numerous safe and effective vaccine are available since 1982 yet infection in adults is dilemma for the whole population [45-47]. In theory, the ideal outcome for the chronic infection is complete elimination of HBV with hepatitis B antigen (HBs) loss, seroconversion to anti-HBs and elimination of cccDNA (covalently closed circular DNA) from the infected hepatocytes., Yet complete elimination of cccDNA from the infected hepatocytes is impossible with any of the current antiviral drugs. Similarly in our study, the patients were tested for the viral load after the completion of therapy, in which most of the patients responded to the treatment so significant fall was noted in viral load of those patients [48].
Peg-IFN is commonly used antiviral drug to treat CHB since 2005 and is almost identical to conventional IFN. Recently a lot of work has been done on this therapy. It has an important role in suppression of virus replication and can lead to HBeAg seroconversion or HBsAg clearance, and normalize the serum ALT level and reduce the risk of cirrhosis and HCC [39,42-44,49]. In last five years peg-IFN has attained interest because of the result of phase ш clinical trials. These trails demonstrated that peg-IFN therapy has more significant result than the standard IFN. These trials also revealed that unlike NAs, peg-IFN does not show antiviral resistance. Because of the reason that peg-IFN is given to the patient for limited time once a week in dose of 180g leading to high rate of seroconversion. However, standard interferon is given to the patient thrice a week in a dose of 4.5 million units [41]. In Taiwan 06 months of peg-IFN treatment is considered standard treatment in CHB infection. Similarly, in current study all the participants were treated with peg-IFN for 06 months with a dose of 180 g, and in some patients the dose was prescribed by the physician according to their health conditions [50].
Genome wide association study shows that three cytokines (IL28A, IL28B and IL29) were present on the long arm of chromosome no. 19 (q13). Among these cytokines IL28B belongs to IFN l family which induce the viral responses by stimulating interferon stimulating genes (ISGs). In past studies this cytokine was identified as main factor of the immune response, which has significant association with treatment of CHC infection [24,51-58]. Recently on basis of these findings same studies were carried out in CHB patients but these investigations were not quite conclusive. Solely because in CHB it is difficult to predict the most accurate biomarker that has a significant outcome in peg-IFN therapy [24,55,56]. Many investigations have been done to determine the pre-treatment predictor of peg-IFN therapy. These observations lead to the fact that peg-IFN therapy has effective outcome in those patients who have low ALT levels and low viral loads. But clinically this predictions are not helpful for therapeutic purposes. Limited data was available in past reports in CHB infection for the pretreatment prediction of IL28B in peg-IFN treated patients, however, few studies have predicted that IL28B has an important role in SVR in peg-IFN therapy. Based on these findings this association gains a lot of interest of the researchers. Sonneveld and his colleagues reported that peg-IFN therapy had significant outcome in clearance of HBV infection by HBeAg seroconversion or by HBsAg clearance [26]. Lampertico investigated 101 HBV infected individuals and all were treated with IFN / peg-IFN therapies, this study revealed that CC genotype could give better outcome as compare to non-CC genotypes. Thomas in his study also revealed, that patients with CC genotype can clear the virus with increased rate. These reports demonstrated that IL28B as a pretreatment predictor. In line with previous studies, we also found rs12979860 CC genotype frequently in responder patients than in non-responder patients (P = 0.03). Galmozzi conducted meta-analysis studies before 2013, he concluded from these set of studies that IL28B has significant association in desired level result [59,60], while in Mangia¢s report 134 CHB infected individuals were investigated, 85% of them have HBeAg negative patients and rest were HBeAg positive. They all were treated with IFN / peg-IFN for 12-24 months. Mangia conclusion does not support Lampertico and Thomas reports, he stated that IL28B (rs12979860) had no influence on HBsAg loss in aforementioned therapies [61]. In 2011, a report was published in which 609 patients of CHB among Chinese Han population were investigated. This report showed significant correlation between IL28B polymorphism, HBV progression and Hepatic inflammation. This study revealed that IL28B genetic polymorphism can prevent the viral progression from advance stages and can also cure liver inflammation. Furthermore this study also revealed that patients who possess CC genotype can give the desired level outcome [62]. Later in 2012 another report was introduced, in which 205 patients were enlisted from Asian European population. Some patients were treated with lamivudine and some were with peg-IFN. After investigation for IL28B genotyping the proportion of CC, CT, and TT were 77%, 19%, 05%, respectively. The author concluded that favorable IL28B genotype (rs12979860) can give effective outcome and can rapidly lead to HBeAg seroconversion during peg-IFN therapy in both Asian and white population [26]. Recently a retrospective study (2005-2010) was reported on 190 CHB patients and all were treated with peg-IFN therapy for tenure of 48 weeks. IL28B (rs12979860) genotypes were accounted as CC-47%, CT-36%, and TT-15%. This report showed statistically significant CC genotype in responder patients ( P = 0.001) after the therapy [63]. Another study was conducted (2014) on 156 Chinese HBV infected population, this study resulted that CC genotype (rs12979860) was the most frequent genotype compare to other two genotypes (CT TT). Furthermore, this report also revealed that patients who have CC genotype have elevated IL28B protein level which provide defensive contribution to the treatment [64]. In Germany a report was published in 2014, in which 86 CHB patients were investigated and all were treated with peg-IFN. The author concluded that IL28B polymorphism has significant prognostic effect in treatment of CHB infection [65]. The results of the above reports and our current study demonstrated the similar outcome. In our current study 200 CHB patients were investigated and after investigations they were divided into two groups R and NR, patients who responded well to peg- IFN therapy and their viral load became less than 2000 copies IU/ml were put into R (n=104) group, and patients whose response was weak to therapy and their viral load were more than 2000 copies IU/ml were put into NR (n = 96) group. In current report the proportion of rs12979860 CC, CT, TT genotypes were found in both R and NR groups as; (R = 72.1%, 25.0%, 2.9% and in NR = 53.1%, 40.6%, 6.2% respectively; P = 0.02), on statistical analysis a positive association (P = 0.02) were observed in both groups. This study has also shown, that CC genotype of IL28B (rs12979860) were the most frequent genotype and it has better response rate to peg IFN therapy as compared to other two genotypes (CT, TT) in this region. Furthermore this study also revealed, that TT genotype was the least frequent genotype in this region. Non-significant contribution of age and gender-wise distribution was noted in both R and NR groups. This study demonstrates the importance of IL28B polymorphism (rs12979860) as pretreatment predictor in peg-IFN therapy in CHB infection.
This study depicts that, IL28B rs12979860 variant may play role as a predictor of peg-IFN therapy against HBV infection. Moreover, this polymorphism may be used as a pre-treatment predictor of peg-IFN therapy. The results of the current study demonstrate that, CC genotype is the most occurring genotype in this region and it has a strong association in rapid HBV clearance during peg-IFN therapy. According to our hectic estimations, efforts need to be carried out on a broader and larger cohort of CHB patients to reach the desired level results. 200 individuals out of the thick populated zone is not enough, as one third of the population is victim of HBV. But the nature of this virus is complex so we need to evaluate certain more SNPs in order to predict accurate pretreatment response of peg-IFN therapy. However extra efforts are required to elucidate the mechanism involved in this association precisely and accurately.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Idrees M, Khan S, Riazuddin S. Common genotypes of hepatitis B virus. Journal of the College of Physicians and Surgeons–Pakistan: JCPSP, (2004); 14(6): 344-347.
- Zhu R, Zhang H-P, Yu H, Li H, Ling Y-Q, et al. Hepatitis B virus mutations associated with in situ expression of hepatitis B core antigen, viral load and prognosis in chronic hepatitis B patients. Pathology-Research and Practice, (2008); 204(10): 731-742.
- Ali L, Idrees M, Ali M, Rehman I-u, Hussain A, et al. An overview of treatment response rates to various anti-viral drugs in Pakistani Hepatitis B Virus infected patients. Virology journal, (2011); 8(1): 20.
- Li G, Li W, Guo F, Xu S, Zhao N, et al. A novel real-time PCR assay for determination of viral loads in person infected with hepatitis B virus. Journal of virological methods, (2010); 165(1): 9-14.
- Paraskevis D, Haida C, Tassopoulos N, Raptopoulou M, Tsantoulas D, et al. Development and assessment of a novel real-time PCR assay for quantitation of HBV DNA. Journal of virological methods, (2002); 103(2): 201-212.
- Alam MM, Zaidi SZ, Malik SA, Naeem A, Shaukat S, et al. Serology based disease status of Pakistani population infected with Hepatitis B virus. BMC infectious diseases, (2007); 7(1): 64.
- Noorali S, Hakim ST, McLean D, Kazmi SU, Bagasra O. Prevalence of Hepatitis B virus genotype D in females in Karachi, Pakistan. The Journal of Infection in Developing Countries, (2008); 2(05): 373-378.
- Hakim S, Kazmi S, Bagasra O. Seroprevalence of hepatitis B and C genotypes among young apparently healthy females of Karachi-Pakistan. Libyan Journal of Medicine, (2008); 3(2): 66-70.
- Ali M, Idrees M, Ali L, Hussain A, Rehman IU, et al. Hepatitis B virus in Pakistan: a systematic review of prevalence, risk factors, awareness status and genotypes. Virology journal, (2011); 8(1): 102.
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology, (2009); 50(3): 661-662.
- Ali Q, Jamal A, Imran M, Ullah S, Kalam I, et al. Correlation of IL28B rs12979860 genotype and gender with spontaneous clearance of HCV infection: a Pakistani cross-section study. Personalized medicine, (2018); 15(6).
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of hepatology, (2006); 45(4): 529-538.
- Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment; 2006. Copyright© 2006 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA. pp. 130-141.
- Saeed MA, Ahmed N, Ali Q. Frequency of Autoantibodies to Thyroid Peroxidase (TPO) in Patients with Type 1 Diabetes Mellitus. International Journal of Applied Biology and Forensics (2018); 2(1): 171-174.
- Lee WM. Hepatitis B virus infection. New England Journal of Medicine, (1997); 337(24): 1733-1745.
- Di Marco V, Iacono OL, Cammà C, Vaccaro A, Giunta M, et al. The long‐term course of chronic hepatitis B. Hepatology, (1999); 30(1): 257-264.
- Fattovich G. Natural course and prognosis of chronic hepatitis type B. Viral Hepatitis Reviews, (1996); 2: 263-276.
- Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology, (1988); 8(3): 493-496.
- McMahon BJ, Alberts SR, Wainwright RB, Bulkow L, Lanier AP. Hepatitis B–related sequelae: prospective study in 1400 hepatitis B surface antigen–positive Alaska native carriers. Archives of Internal Medicine, (1990); 150(5): 1051-1054.
- Liver EAFTSOT. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. Journal of hepatology, (2012); 57(1): 167-185.
- Ali Q, Kalam I, Ullah S, Jamal A, Imran M, et al. Predictive value of IL-28B rs12979860 variants for peg-IFN, sofosbuvir plus ribavirin treatment of HCV infection in Pakistani population. Personalized medicine, (2018); 15(6): 12.
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nature immunology, (2003); 4(1): 63-68.
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, et al. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nature immunology, (2003); 4(1): 69-77.
- Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nature genetics, (2009); 41(10): 1100-1104.
- Martin MP, Qi Y, Goedert JJ, Hussain SK, Kirk GD, et al. IL28B polymorphism does not determine outcomes of hepatitis B virus or HIV infection. Journal of Infectious Diseases, (2010); 202(11): 1749-1753.
- Sonneveld MJ, Wong VWS, Woltman AM, Wong GL, Cakaloglu Y, et al. Polymorphisms near IL28B and serologic response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology, (2012); 142(3): 513-520. e511.
- Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nature genetics, (2009); 41(5): 591-595.
- Guo Y, Guo H, Zhang L, Xie H, Zhao X, et al. Genomic analysis of anti-hepatitis B virus (HBV) activity by small interfering RNA and lamivudine in stable HBV-producing cells. Journal of virology, (2005); 79(22): 14392-14403.
- Mbarek H, Ochi H, Urabe Y, Kumar V, Kubo M, et al. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Human molecular genetics, (2011); 20(19): 3884-3892.
- Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature, (2009); 461(7265): 798.
- Hamdan HZ, Ziad AHM, Ali SK, Adam I. Epidemiology of urinary tract infections and antibiotics sensitivity among pregnant women at Khartoum North Hospital. Annals of clinical microbiology and antimicrobials, (2011); 10(1): 2.
- Jaroszewicz J. Praktyczne zastosowanie ilościowego oznaczania stężeń antygenu HBs. Medical Science Review-Hepatologia, 1(11): 33-36.
- Papatheodoridis GV. Why do I treat HBeAg‐negative chronic hepatitis B patients with nucleos (t) ide analogues? Liver International, (2013); 33(s1): 151-156.
- Perillo R. Benefits and risks of interferon therapy for hepatitis G. Hepatology, (2009); 49(5 Suppl): 103-111.
- Lampertico P, Viganò M, Colombo M. Why do I treat HBeAg‐negative chronic hepatitis B patients with pegylated interferon? Liver International, (2013); 33(s1): 157-163.
- Ono-Nita SK, Kato N, Shiratori Y, Omata M. Current options for the therapy of chronic hepatitis B infection. Current infectious disease reports, (2001); 3(2): 137-142.
- Liaw Y-F, Lin S-M, Chen T-J, Chien R-N, Sheen I-S, et al. Beneficial effect of prednisolone withdrawal followed by human lymphoblastoid interferon on the treatment of chronic type B hepatitis in Asians: a randomized controlled trial. Journal of hepatology, (1994); 20(2): 175-180.
- Lok AS, Wu P-C, Lai C-L, Lau JY, Leung EK, et al. A controlled trial of interferon with or without prednisone priming for chronic hepatitis B. Gastroenterology, (1992); 102(6): 2091-2097.
- Sung J, Tsoi K, Wong V, Li K, Chan H. Meta‐analysis: treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Alimentary pharmacology & therapeutics, (2008); 28(9): 1067-1077.
- van Zonneveld M, Honkoop P, Hansen BE, Niesters HG, Murad SD, et al. Long‐term follow‐up of alpha‐interferon treatment of patients with chronic hepatitis B. Hepatology, (2004); 39(3): 804-810.
- Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, et al. Peginterferon α‐2a (40 kDa): an advance in the treatment of hepatitis B e antigen‐positive chronic hepatitis B. Journal of viral hepatitis, (2003); 10(4): 298-305.
- Chan HLY, Leung NWY, Hui AY, Wong VWS, Liew CT, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-α2b and lamivudine with lamivudine alone. Annals of internal medicine, (2005); 142(4): 240-250.
- Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. The Lancet, (2005); 365(9454): 123-129.
- Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. New England Journal of Medicine, (2005); 352(26): 2682-2695.
- Lacey L. Review of economic benefits of treating chronic hepatitis B with lamivudine. Journal of gastroenterology and hepatology, (2004); 19(S1): 10-12.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of viral hepatitis, (2004); 11(2): 97-107.
- for Research EO, Liver EAFTSOT. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology, (2012); 56(4): 908-943.
- Liver EAFTSOT. EASL Clinical Practice Guidelines: management of chronic hepatitis B. Journal of hepatology, (2009); 50(2): 227-242.
- Skorka C, Grigorescu-Sido P, Manasia R, Lazăr C, Crişan M. EVOLUŢIA HEPATITEI CRONICE VIRALE B LA COPIL SUB TRATAMENT CU INTERFERON. Clujul Medical, 852.
- Liaw YF, Jia JD, Chan H, Han K, Tanwandee T, et al. Shorter durations and lower doses of peginterferon alfa‐2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology, (2011); 54(5): 1591-1599.
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nature immunology, (2003); 4(1): 63-68.
- Marcello T, Grakoui A, Barba–Spaeth G, Machlin ES, Kotenko SV, et al. Interferons α and λ inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology, (2006); 131(6): 1887-1898.
- Urban TJ, Thompson AJ, Bradrick SS, Fellay J, Schuppan D, et al. IL28B genotype is associated with differential expression of intrahepatic interferon‐stimulated genes in patients with chronic hepatitis C. Hepatology, (2010); 52(6): 1888-1896.
- Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, et al. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology, (2010); 139(2): 499-509.
- Ge D, Fellay J, Thompson A, Simon J, Shianna K. rban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature, (2009); 461(7262): 399-401.
- Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nature genetics, (2009); 41(10): 1105-1109.
- Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology, (2010); 138(4): 1338-1345. e1337.
- Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, et al. Lambda interferon (IFN-λ), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. Journal of virology, (2006); 80(9): 4501-4509.
- Lampertico P, Viganò M, Cheroni C, Facchetti F, Invernizzi F, et al. IL28B polymorphisms predict interferon‐related hepatitis B surface antigen seroclearance in genotype D hepatitis B e antigen–negative patients with chronic hepatitis B. Hepatology, (2013); 57(3): 890-896.
- Galmozzi E, Viganò M, Lampertico P. do interferon lambda 3 polymorphisms predict the outcome of interferon therapy in hepatitis B infection? Authors' reply. Alimentary pharmacology & therapeutics, (2014); 39(10): 1251-1252.
- Mangia A, Santoro R, Mottola L, Fasano M, Minerva N, et al. 1331 lack of association between il28b variants and hbsag clearance after interferon treatment. Journal of hepatology, (2011); 54S525.
- Li W, Jiang Y, Jin Q, Shi X, Jin J, et al. Expression and gene polymorphisms of interleukin 28B and hepatitis B virus infection in a Chinese Han population. Liver International, (2011); 31(8): 1118-1126.
- Boglione L, Cusato J, Allegra S, Esposito I, Patti F, et al. Role of IL28-B polymorphisms in the treatment of chronic hepatitis B HBeAg-negative patients with peginterferon. Antiviral research, (2014); 10235-43.
- Shi X, Chi X, Pan Y, Gao Y, Li W, et al. IL28B is associated with outcomes of chronic HBV infection. Yonsei medical journal, (2015); 56(3): 625-633.
- Domagalski K, Pawłowska M, Zaleśna A, Tyczyno M, Skorupa-Kłaput M, et al. The relationship between IL-28B polymorphisms and the response to peginterferon alfa-2a monotherapy in anti-HBe-positive patients with chronic HBV infection. European journal of clinical microbiology & infectious diseases, (2014); 33(11): 2025-2033.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0