Full Length Research Article
Effect of camel milk lactoferrin against carbon tetrachloride induced hepatic toxicity in Sprague Dawley rats
Nasreen Asghar1*, Muhammad Nasir1, Sana-Ullah Iqbal1, Toheed Ahmad1, Robina Majeed2
Adv. life sci., vol. 6, no. 4, pp. 165-175, August 2019
*- Corresponding Author: Nasreen Asghar (Email: nasreenmalik786@yahoo.com)
Authors' Affiliations
2. Shalamar institute of Health Sciences, Lahore, Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background:Chronic Hepatitis and mortality due to liver cirrhosis is common in Pakistan. Camel milk Lactoferrin has antiviral, anti-inflammatory properties. Nutraceutical foods like camel milk have many uses. Camel milk lactoferrin might be used for the cure of hepatic fibrosis induced by
Methods: Seventy-five (75) male Sprague–Dawley rats were purchased from National Institute of Health Islamabad kept in animal house of UVAS Lahore and randomly divided into 5 groups under completely randomize design. In Each group carbon tetrachloride (CC14) was subcutaneously injected with a mixture of 40%
Results: The study was conducted in two phases, first one was on the isolation and purification of camel milk lactoferrin and the second one was based on efficacy study of lactoferrin in Sprague Dawley rats against the hepatic toxicity induced by carbon tetrachloride
Conclusion: The present study on CCl4 induced hepatotoxicity in Sprague Dawley rats clarifies that camel milk lactoferrin induced significant improvement in serum level of ALP, AST, AST, bilirubin, serum urea and serum Creatinine within the duration of 4 weeks treatment.
Keywords: Carbon tetrachloride; Complete randomized design; Aspartate aminotransferase
Liver is the largest gland and vital organ in the body due to its functionality and without its presence survival is impossible [1]. It performs more than 500 tasks and plays a major role in carbohydrates, proteins, fats, steroids and medicines metabolism. Besides, it is involved in activation, storage and transport of vitamins, minerals and nutrients along with synthesis of non-essential amino acids [2]. Liver produces and excretes bile; converts ammonia to urea and operates as a filter by removing bacteria and debris from blood through the phagocytosis by Kupffer cells. Hepatocytes execute functions like glycolysis (break down of glucose), glycogenesis (storage of glucose as glycogen), glycogenolysis (catabolism of glycogen) and gluconeogenesis (production of new glucose from non-carbohydrates molecules) [3].
According to the present situation in Pakistan, camel milk is not utilized at industrial and commercial scale. The farmer will get annual revenue in billions if camel milk used for industrial and commercial scale. Camel milk is effective against various diseases like hepatitis, cancer, diabetes, and hypertension. Functional foods have important impact on the health of human being. These are called health-enhancing products. Camel milk is very important in all worlds and plays an important role in human nutrition. It has proteins like Lactoferrin that has anti- hepatic, Anti-inflammatory, and anti-carcinogenic properties. Camel milk consumed by lactase deficient people and it has no allergic properties. In India it is used to treat jaundice, problems of sleep, anemia and diabetes. Furthermore, in Russia and Sudan camel milk used for the treatment of various diseases like asthma, jaundice, tuberculosis [4]. Lactoferrin is important immune system of body. It shows antimicrobial properties like (bactericide) and shows anti-viral properties. It was determined as main iron requisite protein in human milk by three (3) independent laboratories [5], because it is extracted from milk, iron containing and structurally & chemically alike to serum transferrin that’s why is named as lactoferrin in 1961. Lactoferrin has 60% sequence similarity with serum transferrin that is why classified as a member of transferrin family [6]The significant higher amount of lactoferrin was found in milk and colostrum [7]. Somehow, minute quantity of lactoferrin was also detected in mucosal secretions [8]. Moreover, neutrophils are important source of lactoferrin in human, plasma lactoferrin originates from neutrophils [9]. It is stored in secondary granules and also found in in tertiary granules in lowest concentration [10]. It is found in blood, plasma and serum in low concentration [11]. In blood concentration of Lactoferrin is 0.02ug/ml to 1.52 ug/ml. Plasma lactoferrin may or may not correlate with neutrophils count.
Its concentration increases during inflammation, infection, excessive intake of iron or tumor growth [12]. Besides human, lactoferrin has been identified in a number of mammalian species like cow, pig, buffalo, and goat, bovine and in camel. Numerous studies have been done to evaluate its biological role (Reiter, 1994).
Camel milk lactoferrin (Lf) is preferred to bovine and ruminant lactoferrin due to its stability and distinct characteristics. Camel milk has not attracted in past research as compared to bovine milk, but now due to its unique characteristics like hepatoprotective, anti-inflammatory and anti-carcinogenic properties of camel milk derived Lactoferrin (Lf) provoke the need of future research to isolate its bioactive components to add value.
Liver has both acute and chronic diseases which have global concern and their treatment has several limitations so the search for new drug to treat liver diseases is ongoing. Chronic Hepatitis and mortality due to liver cirrhosis and hepatocellular carcinoma is common in Pakistan. Cam Camel milk Lactoferrin has antiviral, anti-inflammatory, anti-carcinogenic properties.
Lactoferrin is one of the components of immune system in body besides it has main biological functions, like binding and transportation of iron, it has antimicrobial, antibacterial, antiviral, catalytic and anti-carcinogenic functions and properties. It acts as first line defense mechanism against microbial agents invading the organisms mostly through mucosal tissues .It effects growth and proliferation of a variety of infectious agents including both Gram positive and gram-negative bacteria, viruses, protozoa and fungi [13].
Lactoferrin shows antiviral activity, it acts on human and animal viruses which based on DNA and RNA genomes e.g. Cytomegalovirus, Herpes simplex virus 1and 2, Hepatitis C virus. Mechanism of antiviral activity of lactoferrin involved in its diversion of virus from the target cells. Many viruses get attached to lipoproteins of the cell membrane and then penetrate into the cell. Lactoferrin binds to the same lipoprotein and repelling the virus particles. Iron free Apolactoferrin is more efficient in this function than Hololactoferrin.
Another mechanism of antiviral activity of Lactoferrin is that it binds to virus particles and obstruct their entry into cells such as Hepatitis viruses, this mechanism is also confirmed in Rota viruses. Lactoferrin also suppress virus replication after entering into the cell .This is achieved by affected natural killer cells, granulocytes and macrophages cells.
Antiviral activity of human Lactoferrin was first investigated in mice infected with Polycythemia by inducing strain of Friend virus complex [14]. Both human Lactoferrin and bovine Lactoferrin activities against virus have been studied in 1994 [15]. Both Lactoferrin act in early phase of viral infection by blocking the cellular receptors or by directly binding to virus particles thus preventing virus entry into the cells. Bovine Lactoferrin has higher antiviral activity than human Lactoferrin [16].
Lactoferrin also prevents HBV infection in cultured cells and it is different from HCV because cell pretreatment with Lactoferrin was required to inhibit HBV infection.
The liver is the main organ which is responsible for metabolism and detoxification of most of components that enter in the body (Nunez 2006). Carbon tetrachloride (CCI4) is very toxic chemical agent and used to induce liver damage experimentally. On histopathological examination the sectioning of liver tissues reveal the CC14 induced fibrosis, narcosis, and hepatocarcinoma [17]. The number of infiltrated neutrophils, Kupffer cells, lymphocytes, macrophages, and natural killer cells are significantly increased after liver injury induced by CCI4. It induced activation of liver resident macrophages and chemo attraction of extra hepatic cells like neutrophils and lymphocytes [18]. Then activated macrophages are released and leads to liver fibrosis, inflammation and injury [19]. When liver damage occurred then famous chemical drug is limited to treat this condition. So, interest concerned the use of alternative medicine has been arisen for the treatment of hepatic diseases. Camel milk has nutraceutical value due to the presence of specific peptides and proteins like Lactoferrin which has anti-inflammatory, hepatoprotective and anti- hepatocellular carcinoma properties. Furthermore camel milk can be stored at room temperature longer period than the milk obtained from other animals [20]. Camel milk can be consumed by lactase deficient people and it has no allergic properties. Furthermore, in India it is used to treat jaundice, problems of sleep, asthma, anemia and diabetes. Camel milk has unique characteristics which make its uses extensively in the field of medicine as anti- diabetic, hepatoprotective, anti-microbial, anti-tumor, and anti- inflammatory properties but due to lack of previous research demonstrating the protective effect of camel milk derived lactoferrin against carbon tetrachloride induced toxicity in rats was the main reason of this experimental research.
Anti –microbial properties of camel milk were also investigated [21]. Camel milk has anti-toxic effect against cadmium chloride [22]. Although Khan and Alzohainy studied the protective effect of camel milk against hepatic toxicity induced by CCI4 but biochemical parameters like kidney and lipoprotein profiles not investigated fully. So, in the present study will investigate the protective effects of camel milk Lactoferrin against CCI4 induced hepatotoxicity in rats by assessing liver and kidney functions, lipid profiles and histopathology of liver tissues.
Lactoferrin has anti-carcinogenic properties because it regulates the multiple signaling pathways to impart cytotoxic effects on cancer cells. In natural form Lactoferrin is partially saturated with iron and can be fully saturated with iron from external environment. Camel milk Lactoferrin has antiviral, anti-inflammatory, and anti- carcinogenic properties. Lactoferrin acts as (half Transferrin) iron transporting protein and (half Lactoferrin) iron binding protein [23]. Carbon tetrachloride (CCI4) is a highly toxic chemical agent used to induce damage in liver experimentally. CCI4 cause fibrosis, steatosis and hepatocellular carcinoma [24]. Its toxic effect occurs due to trichloromethyl radical formed in the presence of oxidative stress by unstable metabolic intermediate [25].
Study Plan
Present study was conducted in the department of Food Science & Human Nutrition. Camel milk project Lab, Quality Operations Laboratory and Institute of Biochemistry and Biotechnology at University of Veterinary and Animal Sciences, (UVAS) Lahore. In this study, camel milk lactoferrin was extracted and its efficacy was evaluated against CCl4-induced hepatic injury/fibrogenesis in Sprague Dawley rats .
Procurement of raw materials
Camel milk was procured from local market. Milk samples were collected in sterile screw bottles placed in cool boxes (4ᵒC) till transported to the laboratory. Camel milk was defatted and stored at 4ᵒC. The other raw materials were obtained from local market of Lahore. Diagnostic Kits for serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphate (ALP), cholesterol were purchased from Sigma-Aldrich Germany, olive oil was obtained from local market and carbon tetrachloride purchased from E.Merck, Darmstatdt, Germany.
Isolation and purification of Lactoferrin
Isolation and purification of lactoferrin was performed by following method:
a. Preparation of skim milk and whey
In order to prepare skim milk, camel milk was centrifuged at 5,000xg for 30 mint at 4°C and fat layer removed after that skim milk boiled at 50ᵒC and 1 M acetic acid added to maintain the pH at 4.6 allowing it to stand for 1h at 4ᵒC, and casein separated through centrifugation at 10,000xg for 30 minutes. The remaining liquid was Whey, which was neutralized by 1 M NaOH at pH 7.0 after that 80% (1.5M-2.0M) of Ammonium sulphate was added and centrifuged again at 10,000xg for 30 mint at 4ᵒC to remove the precipitates of camel milk lactoferrin.
b. Lactoferrin detection
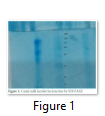
Isolated Lactoferrin was purified by dialysis through dialysis bag against phosphate buffer. Dialysis done for 48hrs.Ammonium sulphate was salted out from lactoferrin liquids. After that purified lactoferrin was run through SDS-PAGE, and EZ Tm-Prestained Protein Ladder marker was put side by side of the gel. Lactoferrin has 80KDa molecular weight and confirmation done by ladder bands shown on the gel. After that lactoferrin was freeze dried at -50 C and stored at -80ᵒC.
c. Lactoferrin determination
Milk Lactoferrin concentrations were measured using a commercially available NOVA Camel Lactoferrin (clf) ELISA kit.
d. Efficacy studies
The efficacy studies of camel milk against hepatic injury/fibrogenesis were conducted through 75 rats. Whole study was piloted consistent with the institutional animal care protocols at the University of Guidelines for the ethical treatment of laboratory animals. Animals were decapitated under chloroform anesthesia and were treated carefully with all efforts to minimize sufferings.
e. Housing of rats
Healthy male Sprague-Dawley rats (albino) aged 7 weeks weighing between 150-250g were purchased from UVAS, Lahore. They were then kept in animal house at UVAS, Lahore. Rats were fed on basal diet for seven days to acclimatize the standard laboratory conditions before study.
f. Establishment of a rat model
Seventy-five (75) male Sprague–Dawley rats were divided under completely randomized design (CRD) into five groups: the group 1 (n=15), the group 2 (n=15), the group 3 (n=15), the group 4 (n=15) and the group 5 (n=15). In group (2-5) carbon tetrachloride (CC14) was intraperitoneally injected with a mixture of 40% CCI4 (a mixture of pure CC14 and sterile olive oil) at 200 ul/100g body weight as single dose, 48 hrs, before the start of treatment or 0 day. After 48 hrs, rats were considered hepatic injured except the (+) control group M1 (Khan and Alzohairy).
Among these five groups, four groups 2 –5 were supplied with standard diet plus Lactoferrin (in different concentrations and doses) orally, while control group was provided only standard diet throughout the efficacy study (30 days). Daily feed and water intake monitored and cages were cleaned regularly. Body weight was recorded before decapitation throughout the experimental period.
Details of these groups are as followed:
Group 1 (+ve control): +ve control group did not injected with CC14, treated with standard diet only.
Group 2 (-ve control): -ve control group was injected by 200 UL/100g body weight. CC14 mixture (CC14 and olive oil in the ratio 2:3) as single dose 48 hrs. Before the commencing treatment and treated by standard diet only.
Group 3 (30mg/kg/b.wt): was injected by 200UL/100 g body weight .CC14 mixture (CC14 and olive oil in the Ratio 2:3) as single dose 48hrs before commencing of treatment and provided with standard diet plus 30 mg/kg body weight /day camel Lactoferrin.
Group 4 (60mg/kg/b.wt): was injected by 200UL/100 g body weight .CC14 mixture (CC14 and olive oil in the Ratio 2:3) as single dose 48hrs before commencing of treatment and provided with standard diet plus 60 mg/kg body weight /day camel Lactoferrin.
Group 5 (90mg/kg/b.wt): was injected by 200UL/100 g body weight .CC14 mixture (CC14 and olive oil in the Ratio (2:3) as single dose 48hrs before commencing of treatment and provided with standard diet plus 90 mg/kg body weight /day camel Lactoferrin. (Khan and Alzohairy, 2011).
The Standard diets confined fixed amount of oil 10%, protein 10%, cornstarch 65%, and cellulose fiber 10%, mineral 3%, and vitamins 1%. Temperature (23±2ᵒC) and humidity 55±5 % retained throughout the experimental period with 12- hrs. Light dark cycle. Feed and water intake were monitored on daily basis, while body weight wa recorded weekly throughout the experimental period.
Five rats (half of the overnight fasted) from each group decapitated at the start of the study to get base line values whereas, five rats were decapitated with 2-weeks interval until end of the study. Blood and organs collected for further analysis. Organs, like heart, liver, left right kidney, pancreas and lungs were removed, cleaned and weighed to calculate organ to body weight ratio. Blood samples were collected through cardiac puncture and both simple as well as EDTA coated tubes were used for blood collection.
g. Samples collection
Blood and organ sample was collected for further analysis. Organs like heart, liver, kidney, pancreas, spleen and lungs were removed, cleaned and weighed to calculate organ to body ratio. Blood samples were collected through cardiac puncture and both simple as well as EDTA coated tubes were used for blood.5 ml blood sample was taken. Blood sampling and histopathology done.
h. Data analysis
The data was analyzed through analysis of variance (ANOVA) to determine the level of significance. Statistical significance was defined as P ≤ 0.05. Means were compared for significance difference using Duncan’s Multiple Range test (DMRt). Costat-2003, Co-Hort, version 6.303 software was used for analysis purpose.
The study was conducted in two phases, first one was on the isolation and purification of camel milk lactoferrin and the second one was based on efficacy study of lactoferrin in Sprague Dawely rats against the hepatic toxicity induced by carbon tetrachloride to explore the nutraceutical worth of camel milk lactoferrin against hepatotoxicity and hepatic injury as it was hypothesized that it is effective against fibrogenesis.
Isolation and purification of Lactoferrin
Isolation and purification of lactoferrin was performed by following methods:
Preparation of skim milk and whey: In order to prepare skim milk, camel milk was centrifuged at 5,000xg for 30 mint at 4°C and then the obtained fat layer was separated with the purpose of preparing whey, the casein was precipitated by optimization of pH to 4-5 with 1M acetic acid, allowing it to stand for 16h at 4C, and the whey was separated by centrifugation at 10,000xg for 30 mint [26].
For isolation and purification of various bioactive components, different methods were performed and optimization of different key parameters was done. At the end, the most appropriate and viable method was adopted to get most purified and maximum yield of products. Lactoferrin was precipitated by 80% concentration (1.5M-2.0M) of Ammonium sulphate.
Lactoferrin Detection
Isolated Lactoferrin was purified by dialysis through dialysis bag against phosphate buffer. Dialysis done for 48hrs.Ammonium sulphate was salted out from lactoferrin liquids. After that purified lactoferrin was run through SDS-PAGE, and ladder was put side by side of the gel. Lactoferrin has 80KDa molecular weight and confirmation done by ladder bands shown on the gel. After that lactoferrin was freeze dried at -20 C and stored.
Lactoferrin Determination
a. Alanine Aminotransferas (ALT) Test
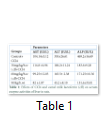
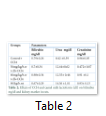
The analysis of variance for changes in ALT (IU/L) to assess the effect of groups and study interval in CCl4 induced hepatic injured rats is presented in Table 2. It illustrates that serum ALT levels in four group (-ve control, 30mg/kg/b.wt,60mg/kg/b.wt,90mg/kg/b.wt) varied significantly from each other with treatment (p=.0000) and interaction of treatment with time (p=.0000) while there was a non-significant difference with time interval (p=.8383). The mean value of ALT(IU/L) that different treatment of lactoferrin lowered ALT concentration in 30mg/kg/b.w,60mg/kg/b90mg/kg/b.wt and there was difference in means according to time intervals in the beginning ALT (IU/L) concentration in all groups was significantly same but varied significantly different after 15 days interval (p=0.0000) and 30 days intervals (p=0.0000). The mean values of ALT(IU) were lowered for 30mg/kg/wt,60mg/kg/b.wt and 90mg/b.wt 189.2 ±12.27 to 186.2 ±11.24 ,171.5± 11.35 to 163.5± 2.38,194± 20.73 to 45.2 ±8.10 respectively but the mean value of ALT(IU) for control group was increased during whole study which was 150 ±26.61 to 350 ±26.61.
b. Aspartate Aminotransferas (AST) Test
The analysis of variance for changes in AST (IU/L) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats is presented in Table 2. It illustrates that serum AST levels in four groups (-ve control, 30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) varied significantly from each other with treatment (p=.0000) and with time interval(p=.0000) while there was also significant difference with interaction of treatment with time interval (p=.0000). The mean value of AST(IU/L) were increased 86.14±5.17 to 104.14±5.12, 90.72±9.36 to 114.8±4.96 for control group and 30mg/kg/b.wt group but lowered 86.55±6.74 to 99.25± 12.03, 86.14±5.02 to 82±1.87 for 60mg/kg/b.wt and 90mg/kg/b.wt respectively at the end of 30 days study.
c. Alkaline phosphatase (ALP) Test
The analysis of variance for changes in ALP (IU/L) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum ALP(IU/L) levels in four groups (-ve control, 30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) varied significantly from each other with treatment (p=.0000) and interaction of treatment with time (p=.0000) while there was also significant difference with time interval (p=.0000).The mean value of ALP(IU/L) were increased 169.2±16.69 to 469.2±16.69, 176.6±13.18 to 183.6±9.28 for control group and 30mg/kg/b.wt group, but ALP concentration were lowered 174.5±13.77 to 171.25±10.30 for 60mg/kg/b.wt and 90mg/kg/b.wt respectively at the end of the 30 days treatment.
Generally, measurement of ALT.AST and ALP level decreased with different treatment of camel milk lactoferrin given at different time interval. These results are supported by [22].
The analysis of variance for changes in Creatinine (mg/dl) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum Creatinine (mg/dl) levels in four groups (-ve control, 30mg/kg/b.wt,60mg/kg/b.wt, 90mg/kg/b.wt) was non-significant from each other with treatment (p=0.1135) and with time interval (p=0.3223) and also there was a non-significant difference with interaction of treatment with time interval (P=.1983). There was difference in means according to time intervals. After induction of liver injury the mean Creatinine (mg/dl) in 30 mg/kg/b.wt group was 0.78±0.27 which statistically decreased to 0.472±0.07 after 30 days study interval. While there was a non-significant decline observed in Creatinine level of 60mg/kg/b.wt group and 90 mg/kg/b.wt group in from 1.02±0.14 to 0.91±0.1 mg/dl and from 1.05 ±0.14 to 0.93±0.13 respectively during 30 days of study duration. The Creatinine level varied from 1.01±0.01 to 0.96± 0.05 in control group.
d. Creatinine Test
The analysis of variance for changes in Creatinine (mg/dl) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum Creatinine (mg/dl) levels in four groups (-ve control, 30mg/kg/b.wt,60mg/kg/b.wt, 90mg/kg/b.wt) was non-significant from each other with treatment (p=0.1135) and with time interval (p=0.3223) and also there was a non-significant difference with interaction of treatment with time interval (P=.1983). There was difference in means according to time intervals. After induction of liver injury the mean Creatinine (mg/dl) in 30 mg/kg/b.wt group was 0.78±0.27 which statistically decreased to 0.472±0.07 after 30 days study interval. While there was a non-significant decline observed in Creatinine level of 60mg/kg/b.wt group and 90 mg/kg/b.wt group in from 1.02±0.14 to 0.91±0.1 mg/dl and from 1.05 ±0.14 to 0.93±0.13 respectively during 30 days of study duration. The Creatinine level varied from 1.01±0.01 to 0.96± 0.05 in control group.
e. Bilirubin Test
The analysis of variance for changes in Bilirubin (mg/dl) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum Bilirubin (mg/dl) levels in four groups (-ve control,30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) was non-significant from each other and also non-significant difference with time (p=.0828) while interaction of treatment with time interval (p=.2664) was non- significant. There was difference in means according to time intervals. The values of bilirubin (mg/dl) for all groups were non-significant throughout the study. The maximum value for bilirubin was observed 0.7± 0.34, 0.98±0.34, 0.674±0.29 for 30mg/kg/b.wt,60mg/kg/b.wt and 90mg/kg/b.wt respectively which statistically same with control group 0.794 ±0.263.
f. Urea Test
The analysis of variance for changes in Urea (mg/dl) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum Urea (mg/dl) levels in four groups (-ve control, 30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) varied significantly from each other with treatment (p=.0000) and with time interval a (p=.0093) while there was a significant difference with interaction of treatment with time interval(p=.0203). Clear difference noted in means according to time intervals. The mean value urea mg/dl was significantly differ from control group .the mean values urea were statistically same 12.44±0.62, 12.55± 0.46, 14.56 ±a1.01 for 30mg/kg/b.wt,60mg/kg/b.wt and 90 mg/kg/b.wt but the mean value urea 8.42 ±0.39 was statistically different for control group.
g. Liver Histopathology
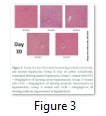
Histopathology results of present study exposed that CCl4 consequences prominent hepatic steatosis, hepatic cord rupture and necrosis. Post treatment of camel milk lactoferrin reduced the severity of CCl4-induced liver intoxication. Fatty change and necrosis were improved in the histological sections of camel milk post treated rats. Photomicrographs regarding histopathological analysis of liver specimens of Sprague Dawely rats for various treatments at different time interval are given below. Each tissue specimen is stained with haematoxylin and eosin. Histopathology results of present study exposed that CCl4 consequences prominent hepatic steatosis, hepatic cord rupture and necrosis. Post treatment of camel milk lactoferrin reduced the severity of CCl4 –induced liver intoxication. Fatty change and necrosis were improved in the histological sections of camel milk post-treated rats. Photomicrographs regarding histopathological analyses of liver tissue specimens of Sprague Dawely rats for various treatments at different time interval are given above. Each tissue specimen is stained with haematoxylin and eosin. Lactoferrin is a main bioactive component isolated from camel milk, having numerous properties to cure hepatitis and liver cirrhosis.
Figures & Tables
Camel milk represents the following bioactive components like Lactoferrin, lysozyme, lactoperoxidase and insulin like protein. Lactoferrin is isolated from colostrum of milk and the first protein of Transferrin family which shows iron binding properties. Casein and whey are the main components ranges between 1.63-2.76% and 0.63-0.80% of camel milk respectively. While, composition whey proteins of camel is quit resembling human milk but different than bovine milk, as camel and human milk are lacking beta –lacto globulin and abundant in alpha-lactalbumin. Lactoferrin has the ability to bind with iron and transfer into the cells and regulate the le free iron levels in blood and external secretions.it is present in milk of human & other mammals, in the all exocrine secretions of mammals like saliva, bile, tears, nasal, tracheal and genital secretions and also found in blood, plasma and neutrophils. Its concentration varies in milk from (>7mg/ml) in colostrum to (>1mg/ml) in mature milk, saliva (>7-10ug/ml), cow’s milk (>20-200ug/ml), seminal plasma (> 0.4-1-9mg/ml), [27]. Lactoferrin shows antiviral activity, it acts on human and animal viruses which based on DNA and RNA genomes e.g. Cytomegalovirus, Herpes simplex virus 1and 2, Hepatitis C virus. Mechanism of antiviral activity of lactoferrin involved in its diversion of virus from the target cells. Although, several methods to establish a model of liver fibrosis and hepatic toxicity have been tried, carbon tetrachloride CCl4 a highly toxic chemical agents, has been extensively used in new drug development for liver diseases to induce well –define model of hepatic centrilobular necrosis subsequently hepatic fibrosis/fatty liver .This experimental models in rats have been used since 1936,because hepatic responses shown to be superficially similar to human cirrhosis. This phenomenon was confirmed by histological changes.
Present study indicated that camel milk lactoferrin reduced the inflammation caused by CCl4 which might lead to the protection of liver injury. Liver damage is the initial factor of hepatic injury and can be assessed by biochemical assays. Transaminases liver enzymes (AST and ALT), And ALP are significant for biological processes, and are reliable markers of liver function .The analysis of variance for changes in ALT (IU/L) to assess the effect of groups and study interval in CCl4 induced hepatic injured rats illustrates that serum ALT levels in four group (-ve control, 30mg/kg/b.wt,60mg/kg/b.wt,90mg/kg/b.wt) varied significantly from each other with treatment (p=.0000) and interaction of treatment with time (p=.0000) while there was a non-significant difference with time interval (p=.8383).The mean value of ALT(IU/L) that different treatment of lactoferrin lowered ALT concentration in 30mg/kg/b.w,60mg/kg/b90mg/kg/b.wt and difference noted in means according to time intervals in the beginning ALT (IU/L) concentration in all groups was significantly same but varied significantly different after 15 days interval (p=0.0000) and 30 days intervals (p=0.0000).The mean values of ALT(IU) were lowered for 30mg/kg/wt,60mg/kg/b.wt and 90mg/b.wt 189.2 ±12.27 to 186.2 ±11.24 ,171.5± 11.35 to 163.5± 2.38,194± 20.73 to 45.2 ±8.10 respectively but the mean value of ALT(IU) for control group was increased during whole study which was 150 ±26.61 to 350 ±26.61.
The analysis of variance for changes in AST (IU/L) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum AST levels in four groups (-ve control, 30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) varied significantly from each other with treatment (p=.0000) and with time interval(p=.0000) while there was also significant difference with interaction of treatment with time interval(p=.0000).The mean value of AST(IU/L) were increased 86.14± 5.17 to104.14 ±5.12 ,90.72± 9.36 to 114.8 ±4.96 for control group and 30mg/kg/b.wt group but lowered 86.55 ±6.74 to 99.25± 12.03 ,86.14 ±5.02 to 82 ±1.87 for 60mg/kg/b.wt and 90mg/kg/b.wt respectively at the end of 30 days study. The analysis of variance for changes in ALP (IU/L) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats shows that serum ALP(IU/L) levels in four groups (-ve control, 30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) varied significantly from each other with treatment (p=.0000) and interaction of treatment with time (p=.0000) while there was also significant difference with time interval (p=.0000). The mean value of ALP(IU/L) were increased 169.2±16.69 to 469.2±16.69, 176.6±13.18 to 183.6±9.28 for control group and 30mg/kg/b.wt group, but ALP concentration were lowered 174.5±13.77 to 171.25±10.30 for 60mg/kg/b.wt and 90mg/kg/b.wt respectively at the end of the 30 days treatment.
Generally, measurement of ALT.AST and ALP level decreased with different treatment of camel milk lactoferrin given at different time interval. These results are supported by[22].The analysis of variance for changes in Cholesterol (mg/dl) to assess the effect of groups and study interval in CCl4 induced hepatic injured rats shows that serum Cholesterol (mg/dl) levels in four groups (-ve control, 30mg/kg/b.wt,60mg/kg/b.wt, 90mg/kg/b.wt) non- significantly differ from each other (p=.8788) but significantly different with time intervals (p=.0204).treatment interaction with time interval also non-significant (p=.9913). The mean cholesterol total (mg/dl) values increased for 30mg/kg/b.wt,60mg/kg/b.wt and 90 mg/kg/b.wt was 124.2 ±18.99 to 204.4 ±128.29, 149.5 ±45.42 to 224.5 ±179.98, 131.6 ±19.13 to 201.6 ±129.21 respectively at the end of 30 days. Though these treatments were statistically resembled with each other but distinctive from control group whose the maximum concentration was 294.8±103.95 after 30 days of study.
The analysis of variance for changes in Bilirubin (mg/dl) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum Bilirubin (mg/dl) levels in four groups (-ve control,30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) was non-significant from each other and also non-significant difference with time (p=.0828) while interaction of treatment with time interval (p=.2664) was non- significant .
The analysis of variance for changes in Urea (mg/dl) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum Urea (mg/dl) levels in four groups (-ve control, 30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) varied significantly from each other with treatment (p=.0000) and with time interval a(p=.0093) while there was a significant difference with interaction of treatment with time interval (p=.0203).
The analysis of variance for changes in Creatinine (mg/dl) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum Creatinine (mg/dl) levels in four groups (-ve control, 30mg/kg/b.wt,60mg/kg/b.wt, 90mg/kg/b.wt) was non-significant from each other with treatment (p=.1135) and with time interval (p=.3223) and also there was a non-significant difference with interaction of treatment with time interval (P=.1983). There was clear difference in means according to time intervals. After induction of liver injury the mean Creatinine (mg/dl) in 30 mg/kg/b.wt group was 0.78 ± 0.27 which statistically decreased to 0.472± 0.07 after 30 days study interval. While there was a non-significant decline observed in Creatinine level of 60mg/kg/b.wt group and 90 mg/kg/b.wt groups in from 1.02 ± 0.14 to 0.91 ±0.1 mg/dl and from 1.05 ±0.14 to 0.93 ± 0.13 respectively during 30 days of study duration.
The analysis of variance for changes in Hemoglobin (g/dl) to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum Hemoglobin (g/dl) levels in four groups (-ve control, 30mg/kg/b.wt,60mg/kg/b.wt, 90mg/kg/b.wt) significantly different from each other with treatment (p=.0000) and with time (p=.0031). There was clear difference in means according to time intervals. The hemoglobin level decreases from 11.56±0.49to 10.6±1.17 for 30mg/kg/b.wt while hemoglobin level improved from 12.125±0.69 to12.725±0.85 and 11.9±0.37 to 12.12±0.57 for 60mg/kg/b.wt and 90mg/kg/b.wt treatment after 15 days’ time interval, respectively; while slightly decline tendency was observed after 30 day treatment i.e from 10.6±1.17 to 11.16±0.6312 for 30mg/kg/b.wt and 12±0.57 to 11.64 ±0.69 for 90mg/kg/b.wt .so there was a significant difference among all treatments after various time intervals.
The analysis of variance for changes in WBC x109/l to assess the effect of diet and study interval in CCl4 induced hepatic injured rats illustrates that serum WBC x109/l levels in four groups (-ve control, 30mg/kg/b.wt, 60mg/kg/b.wt, 90mg/kg/b.wt) were non-significantly from each other with treatment (p=.9304) and but with time there is a significant difference (p=.0104) while there was a non-significant difference with interaction of treatment with time interval (p=.9816). Histopathology results of present study exposed that CCl4 consequences prominent hepatic steatosis, hepatic cord rupture and necrosis. Post treatment of camel milk lactoferrin reduced the severity of CCl4 –induced liver intoxication. Photomicrographs, regarding histopathological analyses of liver tissue specimens of Sprague Dawely rats for various treatments at different time interval are given above. Each tissue specimen is stained with haematoxylin and eosin.
The present study on CCl4 induced hepatotoxicity in Sprague Dawley rats clarifies that camel milk lactoferrin induced significant improvement in serum level of ALP, AST, AST, bilirubin, serum urea and serum Creatinine within the duration of 4 weeks’ treatment. And histopathology also showed significant improvement in hepatotoxicity of liver treated with different doses of camel milk lactoferrin.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Ben-Dan I, Shenhav E. Liver Tumor segmentation in CT images using probabilistic methods; 2008. pp. 43.
- Frühbeck G (2008) Overview of adipose tissue and its role in obesity and metabolic disorders. Adipose Tissue Protocols: Springer. pp. 1-22.
- Hall JE Guyton and Hall textbook of medical physiology. 2015; Elsevier Health Sciences.
- Abdelgadir WS, Ahmed TK, Dirar HA. The traditional fermented milk products of the Sudan. International Journal of Food Microbiology, (1998); 44(1-2): 1.
- Groves ML. The isolation of a red protein from Milk2. Journal of the American Chemical Society, (1960); 82(13): 3345-3350.
- Adlerova L, Bartoskova A, Faldyna M. Lactoferrin: a review. Veterinarni Medicina, (2008); 53(9): 457-468.
- Masson P, Heremans J. Lactoferrin in milk from different species. Comparative Biochemistry and Physiology, (1971); (1): 119-129.
- Masson P, Heremans J, Dive C. An iron-binding protein common to many external secretions. Clinica Chimica Acta, (1966); 14(6): 735-739.
- Lönnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annual review of nutrition, (1995); 15(1): 93-110.
- Tomita M, Saito Y, Hayashi T. LaC2 encapsulated in graphite nano-particle. Japanese Journal of Applied Physics, (1993); 32(2B): L280.
- Boettcher B, Kay D, Rumke P, Wright LE. Human sera containing immunoglobulin and nonimmunoglobulin spermagglutinins. Biology of reproduction, (1971); 5(3): 236-245.
- Birgens HS. Lactoferrin in plasma measured by an ELISA technique: evidence that plasma lactoferrin is an indicator of neutrophil turnover and bone marrow activity in acute leukaemia. Scandinavian journal of haematology, (1985); 34(4): 326-331.
- Kirkpatrick CH, Green I, Rich RR, Schade AL. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. Journal of Infectious Diseases, (1971); 124(6): 539-544.
- Lu L, Hangoc G, Oliff A, Chen LT, Shen R-N, et al. Protective influence of lactoferrin on mice infected with the polycythemia-inducing strain of Friend virus complex. Cancer research, (1987); 47(15): 4184-4188.
- HASEGAWA K, MOTSUCHI W, TANAKA S, DOSAKO S-i. Inhibition with lactoferrin of in vitro infection with human herpes virus. Japanese Journal of Medical Science and Biology, (1994); 47(2): 73-85.
- Valenti P, Antonini G. Lactoferrin. Cellular and Molecular Life Sciences, (2005); 62(22): 2576.
- Althnaian T, Albokhadaim I, El-Bahr SM. Biochemical and histopathological study in rats intoxicated with carbontetrachloride and treated with camel milk. SpringerPlus, (2013); 2(1): 57.
- Ramadori G, Saile B. Portal tract fibrogenesis in the liver. Laboratory Investigation, (2004); 84(2): 153.
- Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology, (2004); 39(2): 273-278.
- Omer RH, Eltinay AH. Changes in chemical composition of camel’s raw milk during storage. Pakistan Journal of Nutrition, (2009); 8(5): 607-610.
- El Sayed I, Ruppanner R, Ismail A, Champagne CP, Assaf R. Antibacterial and antiviral activity of camel milk protective proteins. Journal of Dairy Research, (1992); 59(2): 169-175.
- Al-Hashem F. Camel's milk protects against aluminum chloride-induced toxicity in the liver and kidney of white albino rats. Am J Biochem Biotechnol, (2009); 5(3): 98-109.
- Khan AS, Muller J, Sears J. Early detection of endogenous retroviruses in chemically induced mouse cells. Virus research, (2001); 79(1-2): 39-45.
- Junnila M, Rahko T, Sukura A, Lindberg L-A. Reduction of carbon tetrachloride-induced hepatotoxic effects by oral administration of betaine in male Han-Wistar rats: a morphometric histological study. Veterinary pathology, (2000); 37(3): 231-238.
- Recknagel RO, Glende Jr EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacology & therapeutics, (1989); 43(1): 139-154.
- Yoshida S. Isolation of lactoperoxidase and lactoferrins from bovine milk acid whey by carboxymethyl cation exchange chromatography. Journal of Dairy Science, (1991); 74(5): 1439-1444.
- Korhonen H. Antimicrobial factors in bovine colostrum. Agricultural and Food Science, (1977); 49(5): 434-447.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0![]()