Full Length Research Article
Diurnal variation of brain derived neurotrophic factor and its importance
Muhammad Rafiq1,2, Sajed Ali*,3
Adv. life sci., vol. 7, no. 1, pp. 01-04, November 2019
*- Corresponding Author: Sajed Ali (Email: sajed.ali@skt.umt.edu.pk)
Authors' Affiliations
2. Institute of Clinical Psychology, University of Management and Technology, Lahore, Pakistan
3. University of Management and Technology, Sialkot Campus, Sialkot, Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Previous studies have shown the importance of Brain Derived Neurotrophic Factor (BDNF) in different cognitive processes including learning and memory. Some previous studies have showed variations of BDNF protein during the day. But still there is no data that shows any circadian variation of BDNF in the brain of rodents. So, this study was aimed to determine any circadian variation of BDNF protein in brain structures involved in cognitive processes.
Methods: Male Arvicanthis ansorgei diurnal rats were sacrificed at different zeitgeber times (ZT21, ZT17, ZT13, ZT9, ZT5 and ZT1). ZT12 and ZT0 defining lights off and on respectively. The brains were removed and brain homogenates were prepared from hippocampus and cortex tissues. The amount of BDNF protein was assessed using ELISA technique on the brain supernatants.
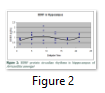
Results: Both the structures i.e. cortex and hippocampus showed a circadian variation of BDNF protein. In cortex, two peaks were observed i.e. at ZT5 and ZT17. Post-hoc analysis showed a significant effect between ZT5 and ZT13 (P<0.05). Hippocampus, also showed two peaks i.e. at ZT9 and ZT21. Post-hoc analysis showed a significant effect between ZT1 and ZT21 (P<0.05).
Conclusion: Our novel results showed that both brain structures of diurnal rodents follow a circadian rhythms of BDNF protein. This study provides a focus for designing experiments and techniques that are based logically how circadian rhythms of different proteins contribute in pathology and how we can treat.
Keywords: Zeitgeber time; Cortex; Hippocampus; ELISA; Arvicanthis ansorgei; Circadian rhythms; BDNF
The Brain Derived Neurotrophic Factor (BDNF) protein belongs to a family of brain proteins called neurotrophin family and it is suggested that BDNF is crucial in important physiological functions including synaptic plasticity, higher cognitive functions and complex behaviours [1]. It has been observed in all areas of the brain especially hippocampus and cortex [2]. Recent studies have indicated that amount of BDNF protein was altered during dark and light periods and BDNF mRNA have also shown variations during the day in the rat brain [3]. BDNF protein daily variations has also been observed in human plasma peak during morning and low during the evenings [4]. In addition to brain areas, hippocampal subfields have also been observed with daily variations of BDNF protein [5].
In all mammals, physiological and behavioural functions are synchronised by the supra-chiasmatic nuclei (SCN) of the hypothalamus [6]. Circadian rhythms of these physiological and behavioural functions are entrained by the environmental cues, called zeitgebers like light [7]. As, BDNF protein has been involved in various processes like synaptic plasticity, learning, and memory. So, due to circadian rhythmicity of BDNF, different physiological and behavioural functions may vary during the day. In this way, it should be presumed that experimental results may vary at different time points during 24 hours. So, this may be important to mention circadian time of the experiments, as manipulation in vivo may have different results in different circadian time.
Indeed, there is no sufficient data on BDNF protein circadian rhythms without any treatment. So, the goal of the current research was to determine any circadian effect on BDNF variations in diurnal rat. So, BDNF protein was quantified at six different time points (zeitgeber times) during 24 hours. Six different zeitgeber times were (ZT21, ZT17, ZT13, ZT9, ZT5 and ZT1). For diurnal rodents, zeitgeber time, ZT12 and ZT1 define the time of activity offset (light off) and onset (light on) respectively.
Ethical consideration: Experiments were performed after institutional approval and in compliance with institutional guidelines that complying with international (National Institute of Health publication, no. 86-23, revised 1985) and national (Service Vétérinaire de la Santé et de la Protection Animale and Ministère de l'Agriculture et de la Forêt) guidelines.
Animals: For obtaining brain structures, young diurnal male Arvicanthis ansogei were used. For acclimatization, subjects were housed on 12/12 light-dark cycle in temp. (20 ± 1°C) and relative humidity (40 ± 2%) with ad libitum food and water.
Procedure: Subjects were sacrificed after euthanasia under CO2 at six different zeitgeber times. Both the brains structures i.e. cortex and hippocampus were quickly removed on ice, and were homogenised in extraction buffer, centrifuged (4000 rmp, 20 min), supernatants were separated and stored at -20°C till quantification of BDNF. BDNF quantification was done by using ELISA kit validated for rat and human BDNF detection (CYT306, Millipore) according to manufacturer’s recommendations [8,9].
Our current data on BDNF showed variations of BDNF protein in cortex and hippocampus of diurnal rats, Arvicanthis ansorgei when subjects were sacrificed and BDNF quantification at six different zeitgeber time (ZT21, ZT17, ZT13, ZT9, ZT5 and ZT1). During 24 hours, both the brain structures showed a variation of BDNF especially at the transitions of light and dark periods. In the cortex, two peaks were observed at ZT5 (5 hours after light on) and ZT17 (5hours after light off). Post-hoc analysis of the data showed a significant effect between ZT5 and ZT13 (P<0.05) and a marginal significant effect was noted among all the zeitgeber times (Fig.1).
In the hippocampus, we also observed two peaks of protein at ZT9 (light period) and ZT21 (dark period). Post-hoc analysis showed a significant effect between ZT1 and ZT21 (P<0.05) and a marginal significant effect was noted among all the zeitgeber times (Fig.2).
Figures & Tables
In this study, our novel data showed a unique variation of BDNF protein in both the brain structures of diurnal subjects when subjects were sacrificed at six different zeitgeber time (ZT21, ZT17, ZT13, ZT9, ZT5 and ZT1). During 24 hours, two peaks of BDNF proteins were observed either in cortex or hippocampus. Interestingly, one peak was in the light period (5hours after light on). This interesting finding may indicate that either light or dark acts like a zeitgeber (stimulus) and BDNF protein is low at both transitions time points. These two peak time points may have importance for better leaning and/or other cognitive tasks and chrono-therapeutic point of view. We used Arvicanthis ansorgei as diurnal rodents active during light as human, and are considered best animals to study diurnal circadian rhythms [10]. BDNF protein was quantified by using ELISA technique that is convenient method for quantifying protein in fluids and tissue homogenates with sensitivity and specificity [11]. Cortical and hippocampal structures were quantified for BDNF protein as these structure are identified with higher levels of BDNF [2]. Again, there is interaction between hippocampus and cortex in perspective to neurochemicals linked with memory and emotions and any alteration in this interaction contributes to psychiatric disorders [12].
Recent studies have shown time of the day effects on neurophysiology and different cognitive processes including learning and memory formation [13]. Studies have also suggested that there are common mechanisms underlie circadian rhythmicity and long-term memory formation [14]. In addition to BDNF, few other brain proteins like that are implicated in cognitive processes have also showed a circadian variations during 24 hours [15].
There is no much data available on circadian variations of BDNF in diurnal subjects especially the in brain structures. This may be due to the fact that there is lack of diurnal model. However, data by Challet et al., showed that Arvicanthis ansorgei can be good subject in diurnal studies. Data from studies on A. ansorgei have shown an interesting circadian variations of activity with two peaks at the transitions of dark and light periods [16]. And it has also been observed that activity is linked with high levels of BDNF protein responsible for increased memory formation [17]. So, two peaks of activity may lead to two peaks of BDNF protein in brain structures. Furthermore, study on corticosterone, two peaks have been observed at the transitions of dark and light periods during 24 hours [18]. This rhythmic variation of corticosterone is parallel with the activity pattern of studied by Slotten.
As BDNF has been shown to be involved in memory performance like some other proteins like Perk1/2 and Perk1/2 is observed to vary rhythmically in rats [19]. The circadian variation of Perk1/2 protein follow the pattern of memory performance in rodents [20]. However, these studies show a single peak during the 24 hours and this may be due type of subjects as they used nocturnal subjects. There is no data available on BDNF protein circadian in relation to synaptic plasticity and memory performance, especially in diurnal animals but results from our data, we may suggest that there can be a parallel effect. At least, this has to be examined. For this, it will be necessary to assess closely the biphasic circadian relation between BDNF and memory performance in diurnal rodents. In addition, our results suggest to perform experiments in specific time of the day, as light as zeitgeber alters the level of proteins during different times of the day. This also suggest to keep maximum possible zeitgebers constant for experiments in vivo that will result in quality and reproducibility of the data.
Authors' Contribution
MR designed and conducted this study, while SA took part in conducting and drafting the manuscript.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Frontiers in molecular neuroscience, (2010); 31.
- Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. European Journal of Neuroscience, (2001); 14(1): 135-144.
- Giese M, Beck J, Brand S, Muheim F, Hemmeter U, et al. Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. Journal of psychiatric research, (2014); 591-7.
- Begliuomini S, Lenzi E, Ninni F, Casarosa E, Merlini S, et al. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. Journal of Endocrinology, (2008); 197(2): 429-435.
- Schaaf MJ, Duurland R, de Kloet ER, Vreugdenhil E. Circadian variation in BDNF mRNA expression in the rat hippocampus. Brain research Molecular brain research, (2000); 75(2): 342-344.
- Hablitz L, Molzof H, Paul J, Johnson R, Gamble K. Suprachiasmatic nucleus function and circadian entrainment are modulated by G protein‐coupled inwardly rectifying (GIRK) channels. The Journal of physiology, (2014); 592(22): 5079-5092.
- van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain research Brain research reviews, (2000); 33(1): 34-77.
- Kozisek ME, Middlemas D, Bylund DB. The differential regulation of BDNF and TrkB levels in juvenile rats after four days of escitalopram and desipramine treatment. Neuropharmacology, (2008); 54(2): 251-257.
- Wei X, Du Z, Zhao L, Feng D, Wei G, et al. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem cells, (2009); 27(2): 478-488.
- Challet E, Pitrosky B, Sicard B, Malan A, Pevet P. Circadian organization in a diurnal rodent, Arvicanthis ansorgei Thomas 1910: chronotypes, responses to constant lighting conditions, and photoperiodic changes. Journal of biological rhythms, (2002); 17(1): 52-64.
- Spiehler VR, Collison IB, Sedgwick PR, Perez SL, Le SD, et al. Validation of an automated microplate enzyme immunoassay for screening of postmortem blood for drugs of abuse. Journal of analytical toxicology, (1998); 22(7): 573-579.
- Jin J, Maren S. Prefrontal-hippocampal interactions in memory and emotion. Frontiers in systems neuroscience, (2015); 9:170.
- Eckel‐Mahan KL, Storm DR. Circadian rhythms and memory: not so simple as cogs and gears. EMBO reports, (2009); 10(6): 584-591.
- Gerstner JR, Yin JC. Circadian rhythms and memory formation. Nature Reviews Neuroscience, (2010); 11(8): 577-588.
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nature neuroscience, (2008); 11(9): 1074-1082.
- Slotten HA, Krekling S, Pevet P. Photic and nonphotic effects on the circadian activity rhythm in the diurnal rodent Arvicanthis ansorgei. Behavioural brain research, (2005); 165(1): 91-97.
- Khabour OF, Alzoubi KH, Alomari MA, Alzubi MA. Changes in spatial memory and BDNF expression to concurrent dietary restriction and voluntary exercise. Hippocampus, (2010); 20(5): 637-645.
- Verhagen LA, Pevet P, Saboureau M, Sicard B, Nesme B, et al. Temporal organization of the 24-h corticosterone rhythm in the diurnal murid rodent Arvicanthis ansorgei Thomas 1910. Brain research, (2004); 995(2): 197-204.
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nature neuroscience, (2008); 11(9): 1074-1082.
- Roth TL, Sweatt JD. Rhythms of memory. Nature neuroscience, (2008); 11(9): 993-994.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0