Disease free and rapid mass production of sugarcane cultivars
Ghulam Zahara Jahangir1*, Idrees Ahmad Nasir2, Muhammad Iqbal1
Adv. life sci., vol. 1, no. 3, pp. 171-180, May 2014
*- Corresponding Author: Ghulam Zahara Jahangir (Email: zahra_jahangir@yahoo.com)
Author Affiliations[Date Received: 13/05/2014; Date Revised: 23/05/2014; Date Published Online: 25/05/2014]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Sacchrum officinarum is acknowledged as a basic source for the production of sugar in Pakistan and worldwide, one of the major constituents of human diet. The presented study optimizes a convenient and successful protocol for in-vitro mass production of sugarcane comprising sixteen cultivars from various sugarcane growing areas all over Pakistan.
Methods: The source plants were sampled randomly from cane growing areas all over the country. Apical region from stalks of germinated plants was taken as explant source. The growth medium used for direct regeneration and multiple shoot formation was same for all constituents but the hormonal supplementations; it comprised of MS basal medium 4.43g/l (MS SIGMA, M 5519), 3% w/v sucrose, and phytagel was added in 0.3% w/v for gelling to support the explant, 0.01mg/l activated charcoal as the carbon source, pH 5.5 to 5.8.
Results: Among various concentrations of BAP used 1.0, 1.5 and 2.0mg/l in growth medium supported efficient regeneration and plenty of lateral shoots in a minimum time span almost in all cultivars. For rhizogenesis, 5.0mg/l of IAA was found to be most efficient among four different concentrations of auxin. Some cultivars have a sufficient endoauxin level and do not need any supplementation for rooting i.e., basal medium supports root induction. For long term maintenance of plant stock, MS broth with 1.0mg/l of BAP was found to be most suitable.
Conclusion: Cytokinin concentrations and plant potential play an equal role in direct regeneration from meristematic tissue.
Keywords: Saccharum officinarum, Tissue culture, Micropropagation, Rhizogenesis
Introduction
Sacchrum officinarum is acknowledged as a basic source for the production of sugar in the Pakistan and worldwide, one of the major constituents of human diet [1]. Besides sugar, almost all of the consumer sweeteners are exclusively produced from sugarcane. Although sugar beet can a substitute to sugarcane for white sugar production but carbohydrates of sugarcane are sweeter in taste than those extracted from sugar beet; and bowing to the technical limitations and huge energy inputs this crop is not popular [2]. Furthermore, sugarcane is also demanded as raw material for a variety of industries like ethanol from cane molasses, alcohol, bagasse, and chip boards [3]. That has made it a second most valued industrial and cash crop of Pakistan [4].
Despite significant production of sugarcane crop (fifth in the world by cane acreage) PFakistan is far below in the hierarchy in sugar recovery, at fifteenth [5]. Many factors can be attributed to low production like lack of proper agricultural management and crop development, abrupt weather change, growers’ financial problems and diseases of crop, lack of adept research in agriculture, unavailability of quality seeds and elite varieties and crop diseases, which is the concerned one, and etc.
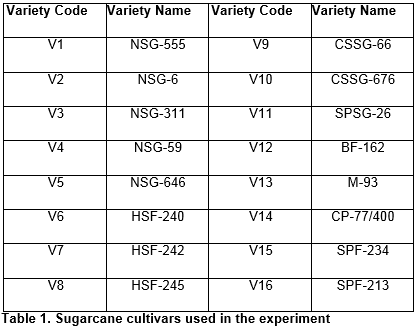
The crop diseases are almost common in cane growing areas all over the Pakistan and are usually caused by crop insects, pests and pathogenic microorganisms. Various pathogens like bacteria, fungi, virus, and mycoplasma greatly reduce the yield of sugarcane crop by attacking the plants during different growth stages [6]. Planting disease free and resistant varieties can sweep out low production issue. Pakistan’s many prominent research institutions and universities are working on sugarcane to develop resistance against certain infections and diseases, but the core problem is the contamination of bioengineered variety when it is multiplied by conventional methods in open field [7]. Plant Tissue Culture technique (a biotechnological tool) is most suitable substitute for conventional methods [8]. It eradicates the risks of contamination during seed production by conventional methods in open field and also ensures rapid multiplication. The exploitation of plant tissue culture technique can certainly reduce the time span for mass production of elite transgenic varieties’ seed; hence their early availability to farmers is made possible. Keeping in view all the potentials and promises of micropropagation, investigation was conducted on sugarcane. The reported study had been carried on sixteen well adopted sugarcane cultivars being grown in Pakistan and was planned to establish an efficient protocol for the mass production of disease free germplasm and its maintenance to be used in further research.
Methods
The source plants were sampled randomly from cane growing areas all over the country. Well adopted cultivars (Table-1) with good agronomic characteristics were screened (data not shown) for the presence of Sugarcane Mosaic Virus (SCMV). Negative plants were cut and sown in the trial field of CAMB, Lahore. Apical region from stalks of germinated plants was taken as explant source. Outer green leaves were removed and stalk was swabbed for sterilization with mercuric chloride (0.01%), absolute ethanol, and autoclaved distilled water one after other in the same sequence. Meristematic tissues of different sizes were excised from inside of yellow immature inner leaf whorls and used as an explant for successive experiments.
The growth medium used for direct regeneration and multiple shoot formation was same for all constituents but the hormonal supplementations [9]; it comprised of MS basal medium 4.43g/l (MS SIGMA, M 5519), 3% w/v sucrose, and phytagel was added in 0.3% w/v for gelling to support the explant, 0.01mg/l activated charcoal as the carbon source, pH 5.5 to 5.8, and the sterilization of the growth medium was achieved by autoclaving for 20 minutes at 105 KPa and 121 °C. For incubation, the photoperiod was adjusted, approximately, at 16 hrs of 2000 lux and 8hrs of dark at 24± 2°C. Inoculation and incubation was done in complete aseptic conditions.
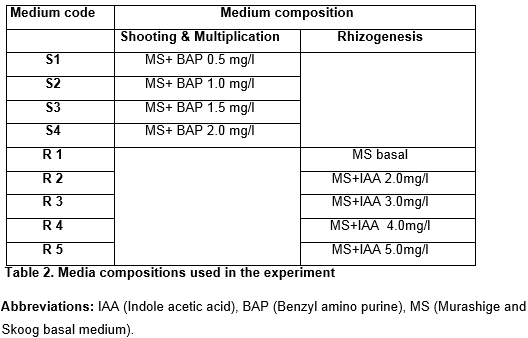
Meristem cultures were kept under the complete dark to avoid phenolics production until they regenerated. Regular shifting or sub-culturing (as per concern) into fresh media was done according to the requirement of the experiment. For the purpose of shoot regeneration from apical meristem and multiple shoot formation, various concentrations of cytokinins (BAP, Table-2) were used. For rhizogenesis, the plantlets were transferred to rooting media on achieving the maximum number of multiple shoots, which comprised of MS media and different concentrations of auxins (IAA, Table-2). Full strength MS medium without any hormonal supplementation was also tested for the purpose. The shoot multiplication medium, for support, contained cotton instead phytagel. Plant stock raised also maintained in MS broth medium with BAP 1.0mg/l in jars and cotton was used. Mean shoot length (in cm) and mean number of shoots per plant was calculated in 100 day old cultures of each variety. Mean root length and mean number of roots per culture was calculated after six weeks of transfer to rhizogenic supplementations.
Results
Direct regeneration
Direct regeneration potential of different sugarcane cultivars, from the shoot apical meristem was examined. For this purpose meristem tissue of different sizes ranging from 3mm to 1.5cm were used. All meristems were implanted vertically in the solid medium. It was rightly noted that meristematic tissue explants with size between 5mm to 1.0cm were capable of shoot regeneration with good survival (Fig. 1A). Time (in days) took to shoot formation increased as the concentration of cytokinin (BAP in mg/l) decreased in growth medium. Generally within two weeks of incubation the pale yellow color of meristem turned greenish along with increase in length approximately up to double of its original length. Tip of the meristem grown over to form a green colored hook like structure (middle culture in Fig.1B) and apical dome was observed to be swelled. After two to four weeks of incubation apical dome formed callus, filled with the meristemoids which supported plantlet regeneration. Hook regenerated green healthy shoot of larger size (right culture in Fig.1B). The MS medium supplemented with four different concentrations of BAP (0.5, 1.0, 1.5, & 2.0mg/l) was used to test the regeneration potential of apical meristem explant. All varieties showed comparative and good results in BAP 1.0 and 1.5mg/l but excellent in 2.0mg/l (Fig.1C) with highest values of mean shoot length in all cultivars (Fig. 2b). Data shown in Figure 2A clearly depict that hormonal supplementation is not the only factor for regeneration but potential of a specific variety is equally affective and each variety response individually.
Multiple shooting
Shoots regenerated from meristem explant were transferred to multiple shoot formation broth (supplemented with different concentrations of BAP) into jars for further growth and proliferation and after 14 weeks of incubation number of shoot regenerated laterally was calculated. Again MS medium supplemented with BAP 2mg/l depicted excellent results for shoot multiplication and good number of lateral shoots produced in this medium (Fig. 1D) as compared to other concentrations of BAP. Once plenty of multiple shoots were formed, the cultures were sub cultured and maintained for longer times in BAP 1.0mg/l (Fig.1E). In all the concentrations of BAP used to investigate multiplication of shoots regenerated from apical meristem, there was great variation in mean number of shoot per explant (Fig. 2C), and mean length of shoots among different varieties (Fig.1F to 1H).
Rhizogenesis:
The regenerated shoots were transferred to root forming media. Full strength MS media without any hormone (basal medium), and along with supplementations of different concentrations of auxins (IAA) were used for root induction. It was observed that some varieties had sufficient endo-auxin level and no supplementations of auxin were required for rooting of regenerated shoots while other needed auxin supplementation even up to 5.0mg/l of IAA (Fig. 1J). Two media worked distinctively best for root formation i.e. basal and MS medium supplemented with 5mg/l of IAA (Fig. 1K). Described supplementation was observed to induce 140 plus roots in cultures of 10 to 15 multiple shoots only (Fig. 3b). Fig.1I shows roots formed in supplements of IAA 3.0, 4.0 and 5.0mg/l from left to right.
Figures
Discussion
BAP has been extensively used by the investigators, in a similar range of concentrations as reported in the presented study, to study the regeneration response of apical meristem of Sacchrum officinarum and have been reported as effective choice. They found 1.5mg/l BAP best responsive in CP 77,400 and the combination of 0.5 mg/l BAP with 0.25 mg/l Kinetin in BL-4 for shoot formation from apical meristems of different sizes as explant. Some other researchers also used four levels of BAP (0.5, 1.0, 1.5 and 2.0 mg/l) and reported its promotive effect on the shoot regenerated from apical meristem of sugarcane cultivars (Co-86032, Co-740 and Co-8014) and found the highest number of leaves on the main shoot on the 1.0 mg/l BAP [10]. Hegde and Kuruvinashetty also used BAP to study the micropropagation of sugarcane [11]. Goasal and his colleagues reported the effect of size of meristem on shoot regeneration through apical meristem in sugarcane varieties Co.J.64, Co.J.83 and Co.P.84-211 [12].
Other researchers also found best results for shoot multiplication in liquid medium with 1.0 mg/l BAP and 0.25 mg/l BAP+ 0.25mg/l Kin in CP 77400 and BL-4 respectively [13]. Punia et al. obtained best results in BAP 1 mg/l for multiplication of shoots regenerated from apical meristem of sugarcane cultivars CoH92 and CoH99. Their findings are in the harmony of the results reported in this study. Similarly the reported effect of phytohormone (auxin) for the rhizogenesis are in close accordance of the results reported in which they used agar solidified rooting medium and reported that MS medium with 5.0 mg/l NAA and 50 g/l sucrose had best rooting efficiency in shoots regenerated from apical meristem [14]. Goasal and his colleagues also obtained rooting in the shoots regenerated from apical meristem by transferring them to liquid MS medium containing 5 mg/l of auxin (NAA) and 70 g/l sucrose [12].
In conclusion, MS basal supplemented with BAP (1.0 and 1.5mg/l) is a good growth medium for the micropropagation of the sugarcane from apical meristem and but is excellent for the purpose when supplemented with BAP 2.0mg/l the same is true for the shoot multiplication. MS basal medium alone and with 5mg/l IAA efficiently support rhizogenesis of the micropropagated plants.
References
- Clarke MA, Edye LA. Sugar Beet and Sugarcane as Renewable Resources; 1996. ACS Publications. pp. 229-247.
- Vaccari G, Tamburini E, Sgualdino G, Urbaniec K, Klemeš J. Overview of the environmental problems in beet sugar processing: possible solutions. Journal of Cleaner Production, (2005); 13(5): 499-507.
- Nguyen TLT, Gheewala SH, Sagisaka M. Greenhouse gas savings potential of sugar cane bio-energy systems. Journal of Cleaner Production, (2010); 18(5): 412-418.
- Akram‐Lodhi AH. A bitter pill? Peasants and sugarcane markets in northern Pakistan. The European Journal of Development Research, (2000); 12(1): 206-228.
- Raza MA, Ashfaq M, Baig IA. Institutional reforms in irrigation sector of Punjab (Pakistan) and their impact on sugarcane productivity. Journal of Agricultural Research, (2009); 47(1).
- Tripathi M, Tripathi S. Fungitoxic evaluation of essential oils of higher plants against sugarcane pathogens in vitro. Annals of Plant Protection Sciences, (2005); 13(1): 223-224.
- Lamkey KR. GMOs and Gene Flow: A Plant Breeding Perspective. Manuscript for the Agricultural Summit http://corn2 agron iastate edu/Lamkey/Publications/PDF/GeneFlow_2002 pdf (abgerufen am 0709 2004), (2002).
- Banerjee N, de Langhe E. A tissue culture technique for rapid clonal propagation and storage under minimal growth conditions of Musa (banana and plantain). Plant cell reports, (1985); 4(6): 351-354.
- Qamar Z, Nasir IA, Husnain T. In-vitro development of Cauliflower synthetic seeds and conversion to plantlets. Advancements in Life Sciences, (2014); 1(2): 34-41.
- Pawar S, Patil S, Jambhale V, Naik R, Mehetre S. Rapid multiplication of commercial sugarcane varieties through tissue culture. Indian Sugar, (2002); 52(3): 183-186.
- Hegde G, Kuruvinashetty M. Standaridization of Protocols for the Micropropagation of Sugarcane. Karnataka Journal of Agricultural Sciences, (2010); 13(4).
- Gosal S, Thind K, Dhaliwal H. Micropropagation of sugarcane an efficient protocol for commercial plant production. Crop Improv, (1998); 2167-171.
- Ali A, Naz S, Siddiqui FA, Iqbal J. An efficient protocol for large scale production of sugarcane through micropropagation. Pakistan Journal of Botany, (2008); 40(1): 139.
- Lee TSG. Micropropagation of sugarcane (Saccharum spp.). Plant cell, tissue and organ culture, (1987); 10(1): 47-55.


![[]Figure 1](http://www.als-journal.com/wp-content/uploads/2014/05/Figure-11.png)
![[]figure 2a](http://www.als-journal.com/wp-content/uploads/2014/05/figure-2a.png)
![[] figure 2b](http://www.als-journal.com/wp-content/uploads/2014/05/figure-2b.png)
![[]Figure 2c](http://www.als-journal.com/wp-content/uploads/2014/05/Figure-2c.png)
![[]Figure 3a](http://www.als-journal.com/wp-content/uploads/2014/05/Figure-3a.png)
![[]Figure 3b](http://www.als-journal.com/wp-content/uploads/2014/05/Figure-3b.png)

