Microbiological stability of chemically preserved apricot pulp
Jabar Zaman Khan Khattak1, Adil Hussain1, Bilal Ahmad1*,Muhammad Fazal–Ul-Rehman1, Zafar Ullah1, Huma Arshad2 and Azhar Hussain3
Adv. life sci., vol. 1, no. 3, pp. 153-159, May 2014
*Correspondence Author: Bilal Ahmad (Email: bilalahmad_271@live.com)
Author Affiliations [Date Received: 10/03/2014; Date Revised: 18/05/2014; Date Published Online: 25/05/2014]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: There exist different methods to preserve the nutrition, color and taste of the fruit pulp for prolonged availability. Bacterial and fungal growth greatly affect the texture and taste of the pulp, if stored for longer period of time. Evaluation of different chemical preservatives to check efficacy and effects on microbial culture growth holds prime importance. The efforts are made in the present investigation to analyze the effect of various concentrations of Sodium Benzoate and Potassium Metabisulphite as preservative on microbial quality of apricot pulp during storage.
Method: The uniformly ripened Halman Apricot pulp was extracted and preserved by chemical preservatives such as Sodium Benzoate (SB), and Potassium Meta-Bisulphite (PMS) at different concentrations. The pulp was investigated for Microbiological parameters i.e. total bacterial count (TBC) and total fungal count (TFC). The inhibitory activity of chemical preservatives was tested periodically by simulating the industrial storage conditions for apricot pulp in the lab (30-42ºC in the dark), for a duration of 60 days.
Results: Significant inhibition in total bacterial count (TBC) was observed in chemically preserved samples. Potassium Metabisulphite was found to be more effective and the highest inhibitory effects on bacterial growth in apricot samples were observed at a concentration of 250mg/250g and 125mg/250g. These were followed by Sodium Benzoate at concentrations of 250mg/250g and 125mg/250g.
Conclusions: This study confirms that the preservatives significantly reduced bacterial and fungal growth in apricot pulp during storage and the pulp was safe for two months without spoilage.
Keywords: Prunusarmeniaca, Chemical preservation, Sodium Benzoate, Potassium Meta-Bisulphite, Total bacterial count, Total fungal count
Introduction
Apricot (Prunusarmeniaca), a member of Rosaceae family, is a stone fruit with an enlarged mesocarp of the ovary wall being the edible part. The pit or stone consists of endocarp whereas the exocarp constitutes the skin of the fruit. The true seed is found enclosed within the endocarp. The fruit is nearly smooth andis of yellow or orange color. The fruit is eaten raw as well as in dried and canned form [1]. World annual production of apricot is approximated to be 579,000 tones [2]. Apricot is cultivated almost all over the world but Turkey, Iran are the world’s largest producers of apricot, accounting for 20%and 10% of world production. Other producers include Italy (5%), Pakistan (5%), Spain (4%), Syria (4%), and United States (3%) France and Morocco (3%) (ERS, 2004). Pakistan is the 4th largest apricot producer of the world with productions reaching 1, 29,652 tones and over an area of 13,758 hectares. Apricot grown in Pakistan is soft and juicy but it has a very short shelf life. Even cold storage cannot lengthen it much. In Pakistan apricot pulp is utilized for manufacturing different value added products.
Apricot fruit is not only consumed in fresh, frozen or dried form but also used for manufacturing pulp, juice, nectar, marmalade, jelly, extrusion products etc. Apricot kernels are also used in aroma perfumes, benzaldehyde cosmetics, oils, and active carbon [3].
Apricot is a perishable fruit having 3-5 days of storage life at optimum conditions and 2-4 weeks at cold storage. The storage life varies with variety. The short storage life of this fruit is due to short time period from commercial ripening to the degradation process characteristic like senescence [4,5].
Different methods are used to preserve apricot pulp but chemical preservation is considered to be the cheapest among numerous methods and is quite common. It is widely used in Pakistan as well. Chemical preservatives are used to prevent microbe caused food spoilage. Apricot pulp is most commonly spoiled by Bacterial and fungal attack which can be controlled by chemical preservatives.
Studies have shown that no single preservative is completely effective against all microorganisms [6]. Calcium (such as Calcium Chloride) is known to conserve the quality of fruits, prevent physiological disorders, reduce the rate of respiration, lessen the solubilization of pectic substance, and maintain the firmness and also to slow down the process of ripening [7]. However, Sodium benzoate (SB) and Potassium metabisulphite (PMS) are more commonly used for long term fruit storage as they have shown better antimicrobial activity [8-10]. SB has been found quite effective against food poisoning, growth and survival of some yeast strains and other spoilage organisms [11,12]. Growth of microorganisms can be inhibited using different concentrations of SB as higher concentrations of SB showed greater antimicrobial effect on different species of Aspergillus [13-15].
Besides having an inhibitory effect on microflora in the stored pulp, addition of preservatives (SB & PMS) may adversely influence its physico chemical characteristics and sensory profile [16]. Addition of preservatives has been known to influence physico chemical characteristics of mango pulp as an increase in acidity; TSS (Brix), reducing sugars and decrease in sucrose content was observed [17]. Codex Standards adopted in 2001 and 2006 have defined maximum levels for the use of these chemicals in fruit preparations including pulp, purees and fruit. These are 1000mg/kg SB as benzoic acid and 500mg/kg PMS as residual SO2. However, careless use of these preservatives is not only a great threat to health and well-being of consumers, but is also well documented to be a primary cause for the appearance of resistant microorganisms thus leading to the occurrence of many emerging food borne diseases [18,19]. Therefore there is a need to optimize dosage levels of these preservatives in apricot pulp. Individual and synergistic effects of these preservatives on various quality attributes under local conditions should also be thoroughly investigated. Therefore a study was conducted in the Department of Agriculture and Food Technology Karakorum international university, Gilgit. Apricot is one of the major fruits in northern areas of Pakistan especially in Gilgit-Baltistan, which is important economically, nutritionally and health point of view. Due to its high moisture content, apricot pulp is highly perishable in nature and has shortest shelf life. So, efforts have been made in the present investigation to improve the shelf life of apricot pulp by using various safe preservatives and to optimize their concentrations as well.
Methods
Procurement of materials
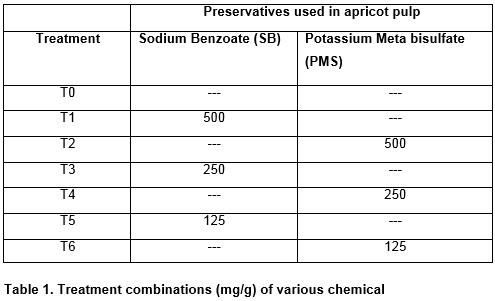
A local apricot variety (Halman) was selected for the study. Ripened fruits were obtained from Shigar valley of Gilgit Baltistan, which were uniform in size, weight and color. The fruit was washed thoroughly with distilled water in a pre-heated tray, to remove unwanted entities like dust, dirt, pesticides residues and surface microflora. Two preservatives sodium benzoate (NaC6H5CO2) (Merck 6290) and potassium metabisulphite (K2S2O5) (Merck 106357) were purchased from dealers of local market.
Pulp extraction, Pasteurization, packaging and storage
After washing, the apricots were dried and processed immediately for pulp extraction. Extraction was done in an electric pulper where pulp was separated from the stones and the obtained pulp was pasteurized in a water bath at a temperature of 82oC for 30 minutes. (At this temperature it is possible to completely kill spore forming bacteria which are sensitive to acidity of apricot pulp, with no changes in physical and chemical attributes) After pasteurization chemical preservatives (SB and PMS) as per treatment combination presented in Table 1 were mixed with the pulp. The treated pulp samples (250g each) were then transferred to sterilized glass bottles and stored under ambient conditions (30-40ºC) in the dark for a period of 60 days and assessed for microbial attributes at an interval of 20 days.
Analysis of Microbiological Parameters of Apricot Pulp
Total microbiological contamination of pulp samples was performed for total bacterial and fungal count of stored pulp samples. One gram sample was acquired from each treated apricot pulp sample by means of aseptic techniques, and placed onto Nutrient medium in autoclaved petri plates. Plates were later incubated (Memmert 100-Germany) for 24-48 hours at 37°C. The pulp samples were analyzed for microbiological quality by considering the total plate count as evaluation index as described by Diliello, 1982 [19]. PDA (potato dextrose agar) was used as media for TFC (Total Fungal Count) and MHA (Mullar Hinton Agar) was used for TBC (Total Bacterial Count) of chemically preserved apricot pulp samples. The experimentation was repeated three times and reported data represents mean values (CFU/g) of these dimensions.
Statistical analysis
Data of present study were analyzed statistically, using analysis of variance [20]. Multiple Range test was applied to assess the difference between means [22]. Significance was defined at p≤0.05. Values are means of three experiments (SD±).
Results
Microbiological analysis of apricot pulp
Total bacterial count (TBC):
The present study was designed to investigate the effect of preservatives in prolonging the storage life of apricot pulp and to find out the optimum concentration required to effectively control fungal and bacterial contamination of stored pulp. For this purpose three different concentrations of each preservative i.e. 125 mg/g, 250 mg/g and 500 mg/g were analyzed. Controlled samples were also analyzed for comparison. The highest inhibitory effects on bacterial growth in apricot samples were observed by PMS at a concentration of 250mg/250g and 125mg/250g. These were followed by SB at 250mg/250g and 125mg/250g.However, none of the concentrations of the two preservatives used completely inhibited the bacterial growth for period of 60 days of storage. The control sample T0 (no preservatives added) showed highest level of contamination in apricot pulp samples after 60 days, whereas, minimum growth was observed in the presence of PMS at various concentrations.
Total fungal count (TFC)
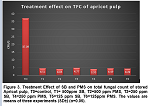
TFC of microbial population is considered as an index of quality of food production. A decrease in fungal growth was observed in the presence of preservatives (PMS and SB). The maximum fungal growth was found in Controlled sample (T0) after 60 days of storage, whereas a considerable reduction was observed in chemically preserved samples. Figures 3 and 4 reveals treatment and storage effects of PMS and SB on fungal growth of apricot pulp. From the present study it can be confirmed that PMS and SB both at a concentration of 500mg/250g were most effective in inhibiting fungal growth in apricot pulp.
Discussion
Chemically preserved apricot samples for TBC were periodically analyzed and a progressive decrease in the growth was observed. Figure 1 and 2 reveals treatment and storage effects of PMS and SB on the bacterial growth of apricot pulp. The decrease in bacterial growth was due to the presence of PMS which is considered to be relatively best inhibitor in apricot pulp. These studies were found to be in accord with previous studies of Akhter et al., Hashmi et al., and Hussain et al., on inhibitory effects of PMS [16, 23, 17]. It is more effective in controlling growth of microorganisms in stored pulp samples .Similar results regarding microbial quality of apricot pulp have also been reported by Sakhale et al., and Hussain et al., [16,21].
In our study decrease in fungal growth was observed in the presence of preservatives (PMS and SB). A previous study on inhibitory effect of preservatives has already shown the reduction of fungal growth in jam samples due to the presence of preservatives [22]. Our results are in complete agreement with Ayub et al., that showed that samples added with 20% sucrose and potassium metabisulphite had overall best results in controlling the microbial population [23]. Brenndor et al., have also reported that addition of sulpher dioxide reduces the microbial population in fruit products [24].
Figures
This study reveals the inhibitory effects of Sodium Benzoate (SB) and potassium metabisulphite (PMS) on microbial growth in the apricot pulp stored under ambient temperature. These preservatives may retain quality attributes especially protein and ascorbic acid contents without any spoilage. Addition of the two preservatives significantly helped in controlling microbial and fungal growth of apricot pulp. The Effect of these chemical preservatives on TBC and TFC of stored apricot pulp, as shown by the present research, may facilitate the development of a safer and feasible storage of apricot pulp at industrial level. We found potassium metabisulphite (PMS) to be more effective in controlling growth of microorganisms in apricot pulp during storage. Both SB and PMS at different concentrations inhibit microbial and fungal growth in apricot pulp and prolong the storage life up to two months without any spoilage. The added preservatives may be also helpful to retain physical, chemical and organoleptic attributes of other value added products of apricot pulp like wine, jams, and squashes etc.
References
- More J, Emmett P. Evidenced‐based, practical food portion sizes for preschool children and how they fit into a well balanced, nutritionally adequate diet. Journal of Human Nutrition and Dietetics, (2014).
- Esengun K, Gündüz O, Erdal G. Input–output energy analysis in dry apricot production of Turkey. Energy Conversion and Management, (2007); 48(2): 592-598.
- Yildiz F. New technologies in apricot processing. Journal of Standard, Apricot Special Issue, Ankara, Turkey, (1994); 67-69.
- Egea M, Martinez-Madrid M, Sánchez-Bel P, Murcia M, Romojaro F. The influence of electron-beam ionization on ethylene metabolism and quality parameters in apricot (< i> Prunus armeniaca</i> L., cv Búlida). LWT-Food Science and Technology, (2007); 40(6): 1027-1035.
- AGAR T, POLAT A. Effect of different packing material on the storage quality of some apricot varieties; 1993. pp. 625-632.
- Utama IMS, Wills RB, Ben-yehoshua S, Kuek C. In vitro efficacy of plant volatiles for inhibiting the growth of fruit and vegetable decay microorganisms. Journal of agricultural and food chemistry, (2002); 50(22): 6371-6377.
- Irfan P, Vanjakshi V, Prakash M, Ravi R, Kudachikar V. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biology and Technology, (2013); 8270-75.
- Sofos J, Busta F. Antimicrobial activity of sorbate [Mostly yeasts and molds]. Journal of Food Protection, (1981).
- Manganelli E, Casolari A. Sensitivity of yeasts to sorbic and benzoic acids and their salts. Industria Conserve, (1983); 58(1): 23-25.
- Lück E. Food applications of sorbic acid and its salts. Food Additives & Contaminants, (1990); 7(5): 711-715.
- Warth A. Resistance of yeast species to benzoic and sorbic acids and to sulphur dioxide. J Food Prot, (1985); 48(7): 564-569.
- Sofos J, Pierson M, Blocher J, Busta F. Mode of action of sorbic acid on bacterial cells and spores. International Journal of Food Microbiology, (1986); 3(1): 1-17.
- Ogiehor I, Ikenebomeh M. Antimicrobial effects of sodium benzoate on the growth, survival and aflatoxin production potential of some species of Aspergillus in garri during storage. Pakistan journal of nutrition, (2004); 3(5): 300-303.
- OGUNRINOLA OA, FUNG DY, JEON IJ. Escherichia coli O157: H7 growth in laboratory media as affected by phenolic antioxidants. Journal of food science, (1996); 61(5): 1017-1021.
- Akhtar M, Zakria M. Incidence of bacterial blight of rice in Pakistan during 2002. Pakistan Journal of Botany, (2003); 35.
- Hussain S, Rehman S, Randhawa M, Iqbal M. Studies on Physico-chemical, microbiological and sensory evaluation of mango pulp storage with chemical preservatives. J Res(Sci), BZ Uni, Multan, Pak, (2003); 1401-09.
- Gibbons A. Exploring new strategies to fight drug-resistant microbes. Science, (1992); 257(5073): 1036-1038.
- Akinpelu DA. Antimicrobial activity of< i> Anacardium occidentale</i> bark. Fitoterapia, (2001); 72(3): 286-287.
- Diliello R. Standard plate count method. Methods in Food and Dairy Microbiology, (1982); 20-29.
- Duncan DB. Multiple range and multiple F tests. Biometrics, (1955).
- Sakhale B, Pawar V, Ranveer R. Studies on Effect of Chemical Preservatives on Keeping Quality of Kesar Mango Pulp. 1: 184. doi: 10.4172/scientificreports. 184 Page 2 of 3 Volume 1• Issue 3• 2012 (SE) and critical differences (CD) at 5% level of significance were worked out for comparison of treatments [7]. Further, confidence intervals were also worked out, (2012).
- Kennedy L. Bobby solar dryers: Their role in post harvest processing. Common Wealth Sci Council, (1985).
- Khattak MMAK, Ayub M, Zeb A, Ullah J. Effect of non-nutritive sweeteners, chemical preservatives and antioxidants on microbial and sensory characteristics of dehydrated guava. Journal of Science and Technology, (2005); 29(1): 63-66.
- 24. Brenndor B, Kennedy L, Oswin C, Trim D, Mrema G, et al. Bobby solar dryers. Their role in post harvest processing Common Wealth Sci Council, (1985).




![[]Figure 4](http://www.als-journal.com/wp-content/uploads/2014/05/Figure-4.png)

