Full Length Research Article
Role of Absent in Melanoma (AIM2) Inflammasome and Proinflammatory Cytokines (Interleukin-18 and 33) in the Pathogenesis of Rheumatoid Arthritis
Sarah Dhaidan1*, Batool Hassan Al-Ghurabi2, Raja Hadi Al-Jubouri3
Adv. life sci., vol. 12, no. 3, pp. 558-563, August 2025
*- Corresponding Author: Sarah Ibrahim Dhaidan (Email: sarahibrahem239@gmail.com)
Authors' Affiliations
2. Immunology, Department of Basic Science College of Dentistry, University of Baghdad, Baghdad – Iraq
3. Al-Turath University College, Baghdad – Iraq
[Date Received: 01/01/2025; Date Revised: 11/02/2025; Available Online: 31/10/2025]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: A chronic autoimmune illness marked by aberrant immune function is rheumatoid arthritis (RA). Absent in melanoma 2 inflammasome (AIM2), is important for the start of the innate immune response. Proinflammatory cytokines are released by the RA's activated inflammasome.
Methods: In this case-control study, 52 participants were divided into 2 groups: 22 had recently been diagnosed with RA, while 30 were healthy subjects. The Disease Activity Score 28 (DAS 28) scored each patient candidate for disease activity. Rheumatologists assess disease activity. Patient and control saliva samples were analyzed using “Enzyme-Linked Immunosorbent Assay (ELISA)” kits to identify AIM2 inflammasome activity and IL-18 and IL-33.
Results: AIM2, IL-18, and IL-33 levels in saliva were considerably higher in the patient group than in the control group.
Conclusion: Elevated levels of the AIM2 inflammasome and the cytokines IL-18, and IL-33 may have a role in the pathophysiology of rheumatoid arthritis and serve as a helpful biomarker for rheumatoid arthritis early detection.
Keywords: Rheumatoid arthritis, AIM2, Inflammasomes, Proinflammatory cytokines
Rheumatoid arthritis is a systemic, chronic autoimmune disease characterized by ongoing inflammation of synovial joints, cartilage degradation, synovial tissue hyperplasia, and joint deformity resulting in functional limitation [1,2]. The proportion of RA patients is rising each year and RA can affect anyone at any age [3,4]. Al-Rawi et al. (1978) estimated a 1% prevalence of RA in Iraq in 1975 [5]. Alkazzaz (2013) noted a rise in the incidence of RA in Iraq, from 1.6% in 2001 to 3.02% in 2011 [6]. A possible explanation for this 1:3 ratio between male and female RA patients is stimulation of the immune system by estrogen [7,8]. Although there is a complicated interplay between genetic and environmental factors [9]. It is believed that smoking, genetics, infections, obesity, periodontal disease and gut microbiota might cause RA, the disease’s exact pathogenesis is still unknown [10]. RA is linked to hyper-gammaglobulinemia and higher levels of acute phase proteins. The synovial membrane contains mononuclear cells, which are then stimulated by T lymphocytes to aid B cells in producing rheumatoid factor [11]. Inflammation and organ damage are hallmarks of a dysregulated immune response, which frequently leads to autoimmune diseases [12,13].
Numerous explanations for chronic inflammation that results in autoimmunity have been suggested. In some autoimmune disorders, inability to regulate inflammasome activation has been identified [13]. Endogenous multimeric protein complexes called inflammasomes cleave proinflammatory cytokines including ‘IL-18 and IL-1β’ in response to infections, tissue injury, and metabolic imbalances [14,15]. Following assembly, the active inflammasome produces mature proinflammatory cytokines by cleaving ‘pro-IL-1β’ and ‘pro-IL-18’ into their component parts. Then proinflammatory cytokines are released [16]. RA’s key proinflammatory cytokines include TNF, IL-1β, IL-6, and IL-18, and the inflammasome sensors include AIM2, pyrin, and NOD-like receptors (NLRs) [17]. AIM2 recruits ASC and pro-caspase-1 when it detects pathogen or host cytosolic double-stranded DNA (dsDNA). This requires ASC and pro-caspase-1 [18].
IL-18, which was originally known as an IFN-inducing factor, is responsible for a number of different functions. These functions include activating T and NK cells to create IFN-, generating a Th1-type immune response, and boosting the proliferative response and cytokine production by activated T cells [19]. In addition to being the root cause of immunologically mediated illnesses, it is responsible for controlling both innate and adaptive immune responses [20]. IL 18 has a variety of regulatory roles in immunological and inflammatory responses and is a sensitive indicator of inflammation [21]. One of the IL-1 cytokine family members, IL-33, mediates the biological effects of its actions via attaching to the ST2 receptor [22]. Additionally, there is a possibility that IL-33 plays a part in the aetiology of rheumatoid arthritis [23,24]. Because of this, the goal of this research was to better understand how the AIM2 inflammasome and proinflammatory cytokines “(IL-18 and IL-33)” contribute to the pathogenesis of RA. The main objective of this study was to understand how the AIM2 inflammasome and proinflammatory cytokines “(IL-18 and IL-33)” contribute to the pathophysiology of rheumatoid arthritis.
Subjects:
In this case-control study, 52 participants were divided into two groups: 22 had recently received a RA diagnosis, while 30 appeared to be in good condition. According to the “Disease Activity Score 28 (DAS 28)”, each candidate in the patient group received a score for disease activity. Disease activity was assessed by a rheumatologist. The College of Dentistry/University of Baghdad Ethical Review Board authorized this research (No. 465 in January 2022). Participants in this study had to meet the 2010 ACR/EULAR inclusion criteria, be older than 20 years old, and not have any infectious diseases like hepatitis, malignancies, cardiovascular complications, or other autoimmune or inflammatory diseases.
Sample size calculation:
The G power 3.1.9.7 tool estimated the sample size with 95% power of research and 0.05 alpha error of probability on a two-sided scale.
Estimation of DAS28:
According to DAS 28, each candidate in the patient group received a score for disease activity. This index includes three measurements: an ESR measure (as a marker of inflammation), a TJC (tender joint count) range of 0-28, and an SJC (swollen joint count) range of 0-28. The following equation was used for calculating DAS28:

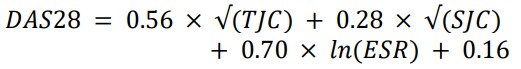
Saliva Samples Collection:
All subjects were instructed to fast for at least an hour, sit comfortably, and discard blood samples before contributing saliva. The participants were also instructed to sit comfortably. The saliva was obtained between 9 and 12 that morning. After continuously rinsing his mouth with sterile water and waiting one to two minutes, up to 5 ml of unstimulated saliva was collected into polyethylene tubes. After collecting saliva, it was centrifuged for 10 minutes at 3000 rpm and stored in Eppendorf tubes at -80 °C.
Measurement of Salivary Biomarkers:
The ELISA kits (Mybiosource/USA and Shanghai/China) were used to measure the concentration of AIM2 inflammasome and proinflammatory cytokines ‘IL-18 and IL-33’.
Statistical analysis:
Statistics were performed using SPSS version 24. Differences between rheumatoid arthritis and control groups were assessed using an independent samples t-test. When data were not normally distributed, the Mann–Whitney U test was applied as a non-parametric alternative. Associations between variables were evaluated using the Pearson correlation coefficient. A p-value ≤ 0.05 was considered statistically significant.
Based on the comparison of 22 RA patients with 30 healthy controls, the findings of this study were determined. Table 1 lists the demographic characteristics of patient group and control group. Age differences among the two study groups weren't significant (p>0.05). The average age of the participants was 46.91±7.49 years, and a significant gender difference (p <0.05) was found between the two study groups. Most of the participants of the RA patients were females: 19 (86%) compared to 3 (14%) male participants. The average DAS28 score for the group of RA patients in our study was 4.83 ± 1.07, considering disease activity at the time of diagnosis.
This study's results indicated a substantial increase in salivary AIM2 levels in the RA group (2.29±0.33) compared to the control group (1.28±0.25), as shown in Table 2.
In the current study, there was significant elevation in mean salivary levels of IL-18 (p < 0.001) and IL-33 (p = 0.001) among RA patients (26.30±4.23 and 338.1±153.0) as compared to the control group (11.40 ± 1.63 and 205 ± 39.37), as observed in Table 3.
The current study showed that there was a significant correlation between DAS28 index at the time of diagnosis and salivary level of AIM2, IL-18 and IL-33 (r=0.467, P=0.029; r=0.589, p=0.004; r=0.500, p=0.018) in RA patient group as shown in Table 4.
Figures & Tables
The prevalence of RA in the population is well recognized, with the fourth and fifth decades of life having the highest occurrence. But the average patient age in the current study was (46.91 ± 7.49), which is generally in line with findings from other studies [25,26]. Additionally, compared to men, females had a higher prevalence of RA, according to this study. These results are comparable with those published by [27]. Studies [6,28-32] conducted in Iraq found that females had greater incidences of RA than males (5.3:1, 4.6:1, 3.7:1, 4.9:1, and 6:1), respectively. The interpretation of a higher prevalence in females may be related to the immunological responses that result from hormonal variations (such as estrogen) between them. These hormones cause women to express superior immune responses than males do, and these responses have a tendency to be more TH2 responses, which are pro-inflammatory, so they may accelerate the onset of autoimmune disorders [32].
Recent studies on the pathophysiology of RA are increasingly focusing on cytoplasmic receptors called AIM2 inflammasomes. According to the current study, in comparison to the control group, the RA patient group's salivary AIM2 level increased. In PBMCs from RA patients, AIM2 gene expression levels were considerably increased, according to a meta-analysis by Afroz and colleagues [33]. However, Baum et al. (2015) found that mice susceptible to arthritis who were AIM2 deficient showed considerably reduced joint inflammation and histological changes [34]. Méndez-Frausto et al. (2020) found that rheumatoid arthritis monocytes produced more IL-1β without AIM2 inflammasome signaling [35]. However, Chen et al. (2020) found that rheumatoid arthritis patients had lower blood AIM2 levels than healthy controls, while “caspase-1, ASC, IL-1β, and AIM2” inflammasome-associated molecules were higher and strongly correlated with CRP and ESR. However, RA patients' FLSs had greater AIM2 levels than OA patients' FLSs did, and inhibiting AIM2 in FLSs prevented FLS proliferation [36]. AIM2 is still a focus for RA treatment, despite the contradictory outcomes. Hypoxia damages nuclear or mitochondrial DNA (mtDNA), which is closer to the respiratory chain and more susceptible to oxidative stress. Raised plasma and synovial tissue mtDNA levels and AIM2 inflammasome activation are seen in rheumatoid arthritis patients [37,38].
As previously reported, the rheumatoid arthritis group had significantly greater salivary IL-18 levels than the control group. RA patients showed greater median IL-18 concentrations than controls, according to Talib et al. (2024) [39]. Shao et al. (2009) found that RA patients had higher blood, synovial fluid, and tissue levels of “IL-18, IL-18R, iNOS, COX-2, and IL-18” biological activity than controls [40]. Proinflammatory cytokines, particularly ‘IL-1β and IL-18’, are cleaved by activated inflammasomes in response to a variety of stimuli, including infections, tissue injury, and metabolic imbalances [14,15]. Deregulation of the AIM2 inflammasome may explain the increased salivary IL-18 in this research. Due to inflammasome growth, pro-IL-1 and pro-IL-18 mature into IL-1 and IL-18, respectively, and are released into the extracellular environment by pyroptosis, activating caspase-1 downstream [41].
The fact that patients' saliva contained more IL-33 than that of control subjects was another significant finding of this investigation. This result is consistent with the [42] research, which confirmed a wide assortment of IL-33 levels in RA patient synovial fluids, which were much higher than those in OA patients. Hong and colleagues also found an increase of IL-33 in RA, suggesting that it may regulate autoantibody production [43]. One of the primary sources of IL-33 in RA is thought to be synovial fibroblasts, which produce large amounts of the protein when stimulated by “TNF-α and IL-1β” [23]. Reduced partial oxygen pressure is a notable feature of the microenvironmental conditions in the inflamed joints of rheumatoid arthritis patients [44-47].
The signaling pathways governing ‘IL-33 expression’, particularly the p38 and ERK pathways, were activated by HIF-1α to stimulate IL-33 expression in RASF. This explains, at least in part, why RA patients' synovial fluids have raised amounts of IL-33, and it also suggests that elevated serum levels of IL-33 may be caused by high levels of TNF [48]. The results of the current investigation demonstrated a strong correlation between salivary AIM2, IL-18, and IL-33 levels and the DAS28 index in the RA patient group. The current finding somewhat accords with a different study by Chen et al. (2020), who found a positive correlation between RA patients' ESR and CRP levels and the H scores for AIM2, ASC, and IL-1 in the afflicted knee synovium. These findings suggest that the AIM2 inflammasome may contribute to the pathophysiology of RA [36]. Furthermore, in rheumatoid arthritis patients, a link existed between salivary IL-33 levels and disease activity. Similar findings were reported by Matsuyama et al. (2010) [49] observed same results, establishing a correlation between DAS28 and serum IL-33 levels. The concentration of IL-33 in synovial fluid was correlated with DAS-28, ESR, and RF in a research including 120 RA patients [50]. This could be an indication of a complex interaction between IL-33 levels and RA patient inflammation. However, Xiangyang et al. (2012) found no association between serum IL-33 levels and other clinical variables including DAS28, ESR, or CRP [51]. The results of the present study suggested a relationship between salivary IL-18 and disease activity. This finding agreed with those of Şahin et al. (2014), who discovered that in RA patients, IL-18 positively linked with DAS 28 scores [52].
Limitation
This study has certain limitations that should be acknowledged. Although a power analysis was performed to determine the minimum required sample size, the number of participants was relatively small (22 patients and 30 controls), which may limit the generalizability of the findings. Additionally, the cross-sectional design restricts causal inference regarding the relationship between AIM2 inflammasome activation, cytokine expression, and rheumatoid arthritis activity. Future studies with larger, more diverse cohorts and longitudinal follow-up are recommended to validate and expand upon these findings.
Acknowledgement
The authors thank all participants in the present study.
Author Contributions
Sarah Ibrahim Dhaidan: Conceptualization, study design, data collection, manuscript drafting, manuscript editing, final approval of the manuscript and technical support.
Batool Hassan Al-Ghurabi: Reviewing and supervision.
Raja Hadi Al-Jubouri: Manuscript review and supervision.
The authors state that they have no competing interests.![]()
References
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. The Lancet, (2016); 388(10055): 2023–2038.
- Xiao F, Han M, Rui K, Ai X, Tian J, et al. New insights into follicular helper T cell response and regulation in autoimmune pathogenesis. Cell Cellular & Molecular Immunology, (2021); 18(6): 1610–1612.
- Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis & Rheumatism, (2011); 63(3): 633–639.
- Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, et al. Global, regional, and national burden of rheumatoid arthritis 1990–2017: A systematic analysis of the Global Burden of Disease study 2017. Annals of the rheumatic diseases, (2019); 78(11): 1463–1471.
- Al-Rawi ZS, Alazzawi AJ, Alajili FM, Alwakil R. Rheumatoid arthritis in population samples in Iraq. Annals of the rheumatic diseases, (1978); 37(1): 73–75.
- Alkazzaz AMH. Incidence of rheumatoid arthritis [2001 to 2011]. The Iraqi Postgraduate Medical Journal, (2013); 12(4): 568–572.
- Tan IJ, Peeva E, Zandman-Goddard G. Hormonal modulation of the immune system—A spotlight on the role of progestogens. Autoimmunity reviews, (2015); 14(6): 536–542.
- Pierdominici M, Maselli A, Colasanti T, Giammarioli AM, Delunardo F, et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunology letters, (2010); 132(1–2): 79–85.
- Aldhaher Z, Al-Ghurabi B, Alwan B. Serum levels of IL-22 and ACPA in patients with rheumatoid arthritis. Journal of PurE and aPPliEd Microbiology, (2018); 12(2): 687–691.
- Jiang Q, Yang G, Xiao F, Xie J, Wang S, et al. Role of Th22 cells in the pathogenesis of autoimmune diseases. Frontiers in immunology, (2021); 12(2021): 1-14.
- Subhi IM, Zgair AK. Estimation of levels of interleukin-1 beta and interleukin-10 in sera of some Iraqi patients with chronic rheumatoid arthritis. Iraqi Journal of Science, (2018); 59(3C): 1554–1559.
- Chang C. The pathogenesis of neonatal autoimmune and autoinflammatory diseases: A comprehensive review. Journal of autoimmunity, (2013); 41(2013): 100–110.
- Yang CA, Chiang BL. Inflammasomes and human autoimmunity: A comprehensive review. Journal of autoimmunity, (2015); 61(2015): 1–8.
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nature Reviews Immunology, (2013); 13(6): 397–411.
- Broz P, Dixit VM. Inflammasomes: Mechanism of assembly, regulation, and signalling. Nature Reviews Immunology, (2016); 16(7): 407–420.
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual review of immunology, (2009); 27(1): 519–550.
- Ting JPY, Lovering RC, Alnemri ES, Bertin J, Boss JM, et al. The NLR gene family: A standard nomenclature. Immunity, (2008); 28(3): 285–287.
- Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science, (2009); 323(5917): 1057–1060.
- Ibrahim MII, Al-Saffar JM. Serum level evaluation of interleukin-18 in obese women with polycystic ovary syndrome. Iraqi Journal of Science, (2018); 59(4B): 1989–1994.
- Al-Bassam WW, Ad’hiah AH, Mayouf KZ. Biomarker significance of interleukin-18 in juvenile idiopathic arthritis. Iraqi Journal of Science, (2020); 61(12): 3200–3207.
- Al Obaidi MJ, Al Ghurabi BH. Potential role of NLRP3 inflammasome activation in the pathogenesis of periodontitis patients with type 2 diabetes mellitus. Journal of Medicinal and Chemical Sciences, (2023); 6(3): 522-531.
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nature Reviews Immunology, (2010); 10(2): 103–110.
- Xu D, Jiang HR, Kewin P, Li Y, Mu R, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proceedings of the National Academy of Sciences, (2008); 105(31): 10913–10918.
- Athari SK, Poirier E, Biton J, Semerano L, Hervé R, et al. Collagen-induced arthritis and imiquimod-induced psoriasis develop independently of interleukin-33. Arthritis Research & Therapy, (2016); 18(1): 1-11.
- Ahmad ST, Joyce MV, Boggess B, O’Tousa JE. The role of Drosophila ninaG oxidoreductase in visual pigment chromophore biogenesis. Journal of Biological Chemistry, (2006); 281(14): 9205–9209.
- Ranade S, Doiphode S. Is there a relationship between periodontitis and rheumatoid arthritis?. Journal of Indian Society of Periodontology, (2012); 16(1): 22-27.
- Bukhari M, Lunt M, Harrison BJ, Scott DGI, Symmons DPM, et al. Rheumatoid factor is the major predictor of increasing severity of radiographic erosions in rheumatoid arthritis: Results from the Norfolk Arthritis Register Study, a large inception cohort. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, (2002); 46(4): 906–912.
- Jassim NAL, Ibrahim DH, Gorial FI. Efficacy and safety of etanercept in severely active rheumatoid arthritis: 6-month, open-label, prospective, observational study from Iraq. Journal of Natural Sciences Research, (2015); 5(2): 120–124.
- Khidhir RM, Al-Jubouri RH. The study of temporomandibular joint disorders and anti-cyclic citrullinated peptide antibodies in serum and saliva of patients with rheumatoid arthritis. Journal of Baghdad College of Dentistry, (2013); 25(Special Issue): 67–71.
- Khalid KB, Humadi YA, Gorial FI, Awadh NI, Mahmood SJ, et al. Evaluation of oral health-related quality of life in a sample of Iraqi patients with rheumatoid arthritis: a case-control study. Journal of Oral Medicine and Oral Surgery, 2024; 30(1): 1-7.
- Taha GI. Involvement of IL-10 gene polymorphism (rs1800896) and IL-10 level in the development of peri-implantitis. Minerva Dental and Oral Science, (2024); 73(5): 264–271.
- Talib EQ, Taha GI. Involvement of interleukin-17A (IL-17A) gene polymorphism and interleukin-23 (IL-23) level in the development of peri-implantitis. BDJ Open, (2024); 10(1): 1-8.
- Afroz S, Giddaluru J, Vishwakarma S, Naz S, Khan AA, et al. A comprehensive gene expression meta-analysis identifies novel immune signatures in rheumatoid arthritis patients. Frontiers in immunology, (2017); 8(2017): 1-12.
- Baum R, Sharma S, Carpenter S, Li QZ, Busto P, et al. Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II–deficient mice. The Journal of Immunology, (2015); 194(3): 873–877.
- Méndez-Frausto G, Medina-Rosales MN, Uresti-Rivera EE, Baranda-Cándido L, Zapata-Zúñiga M, et al. Expression and activity of AIM2-inflammasome in rheumatoid arthritis patients. Immunobiology, (2020); 225(2): 151880.
- Chen Y, Fujuan Q, Chen E, Yu B, Zuo F, et al. Expression of AIM2 in rheumatoid arthritis and its role on fibroblast-like synoviocytes. Mediators of Inflammation, (2020); 2020(1): 1–10.
- Lee SH, Chang DK, Goel A, Boland CR, Bugbee W, et al. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. The Journal of Immunology, (2003); 170(4): 2214–2220.
- Hajizadeh S, DeGroot J, TeKoppele JM, Tarkowski A, Collins LV. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Research & Therapy, (2003); 5(5): 1-7.
- Talib EQ, Taha GI, Ali DM, Al-Hindawi SH, Al-Khayat FAA, et al. The microbial boundaries in peri-implantitis: A review of pathogen-related advances. Folia Medica, (2024); 66(6): 763–769.
- Shao XT, Feng L, Gu LJ, Wu LJ, Feng TT, et al. Expression of interleukin-18, IL-18BP, and IL-18R in serum, synovial fluid, and synovial tissue in patients with rheumatoid arthritis. Clinical and experimental medicine, (2009); 9(3): 215–221.
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature, (2009); 458(7237): 509–513.
- Hu F, Shi L, Mu R, Zhu J, Li Y, et al. Hypoxia-inducible factor-1α and interleukin 33 form a regulatory circuit to perpetuate the inflammation in rheumatoid arthritis. PLoS One, (2013); 8(8): e72650.
- Hong YS, Moon SJ, Joo YB, Jeon CH, Cho ML, et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. Journal of Korean medical science, (2011); 26(9): 1132.
- Peters CL, Morris CJ, Mapp PI, Blake DR, Lewis CE, et al. The transcription factors hypoxia-inducible factor 1α and Ets-1 colocalize in the hypoxic synovium of inflamed joints in adjuvant-induced arthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, (2004); 50(1): 291–296.
- Naughton DP, Haywood R, Blake DR, Edmonds S, Hawkes GE, et al. A comparative evaluation of the metabolic profiles of normal and inflammatory knee-joint synovial fluids by high-resolution proton NMR spectroscopy. FEBS letters, (1993); 332(3): 221–225.
- Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, (1970); 13(6): 769–776.
- Taha GI, Talib EQ, Abed FB, Hasan IA. A review of microbial pathogens and diagnostic techniques in children’s oral health. Sri Lanka Journal of Child Health, (2024); 53(4): 355–359.
- Hollander AP, Corke KP, Freemont AJ, Lewis CE. Expression of hypoxia-inducible factor 1α by macrophages in the rheumatoid synovium: Implications for targeting of therapeutic genes to the inflamed joint. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, (2001); 44(7): 1540–1544.
- Matsuyama Y, Okazaki H, Tamemoto H, Kimura H, Kamata Y, et al. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. The Journal of Rheumatology, (2010); 37(1): 18–25.
- Tang S, Huang H, Hu F, Zhou W, Guo J, et al. Increased IL-33 in synovial fluid and paired serum is associated with disease activity and autoantibodies in rheumatoid arthritis. Journal of Immunology Research, (2013); 2013(1): 1–6.
- Xiangyang Z, Lutian Y, Lin Z, Liping X, Hui S, et al. Increased levels of interleukin-33 associated with bone erosion and interstitial lung diseases in patients with rheumatoid arthritis. Cytokine, (2012); 58(1): 6–9.
- Şahin M, Ugan Y, Tunç ŞE, Akın Ş, Köroğlu B, et al. Potential role of interleukin-18 in patients with rheumatoid arthritis-associated carotid intima-media thickness but not insulin resistance. European Journal of Rheumatology, (2014); 1(4): 135–139.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0







