![]()
Phytotoxicity of Hg and its Detoxification through Microorganisms in Soil
Aatif Amin*, Zakia Latif
Adv. life sci., vol. 2, no. 2, pp. 98-105, February 2015
*-Corresponding Author: Aatif Amin (Email: aatifamin93@gmail.com)
Author AffiliationsDepartment of Microbiology and Molecular Genetics, University of the Punjab, Lahore-54590, Pakistan
[Date Received: 14/01/2015; Date Revised: 23/02/2015; Date Published Online: 25/02/2015]
Abstract![]()
Introduction
Methods
Discussion
Conclusion
References
Abstract
Due to the advent of industrialization, pollution of terrestrial environment by heavy metals has emerged as a great issue. Therefore, it is an urgent need to realize the Hg-induced toxicity in plants and as well as in animals and the harmful effects by the consumption of contaminated nutrition. Mercury is considered as a hazardous contaminant that can be changed into various oxidation states easily and causes many deleterious effects in several physiological processes in both plants and animals. Microorganisms possess two extensively studied Hg-detoxification processes like Mer operon (merTPCFAD) and Met gene which encode the functional proteins for transportation (merT, merP and/or merC, merF), reduction (merA) and a secondary regulatory protein (merD) and sulfhydrylase enzymes (met gene) respectively to modify toxic Hg+2 to nontoxic elemental state (Hg0). Due to the ever increase in Hg-pollution and very little information about its phytotoxic effects and detoxification mechanisms, the authors expect, the present article will make possibility in the provision of a comprehensive literature study about Hg-induced toxicity in plants and its detoxification processes to provoke for advance research in this field.
Key words: Hg-oxidation states, Biogeochemical cycling of Hg, Hg-phytotoxicity, Hg-detoxification, Met gene, Mer operon
Introduction
Mercury (Hg), also known as hydrargyrum or quicksilver is a group IIb element with an atomic weight of 200.59. Its property to remain liquid at room temperature and normal pressure makes it a unique metal. It rarely occurs in pure state in nature but is found most commonly as the ore cinnabar (HgS) [1]. Earlier records indicate that mercury is used in alchemy in China as early as the second century B.C and references to cinnabar mines and the medicinal use of mercury were made by Pliny the Elder in the first century. On average, the level of mercury in earth crust is around ~0.5 ppm but large deposits of Hg are found in the areas of volcanic activity. It has been released into the lithosphere, atmosphere and hydrosphere by geochemical process and therefore it is an important toxic element in the biosphere [2, 3]. Mercury tends to be found in three oxidation states as a Hg(0)(elemental or metallic), Hg22+ (mercurous) and Hg2+ (mercuric ion) [4]. Metallic mercury is relatively nontoxic as compared to oxidized states of Hg due to its low solubility, but it can be modified to highly toxic oxidized forms in vivo by catalase and peroxidase enzymes. The level to which mercury can prove hazardous strongly relies in its oxidation state. Different forms of mercury in chemical terms have been classified [5, 6] as
|
Volatile species |
Hg0 and (CH3)2Hg |
|
Reactive species |
Hg+2, HgX+, HgX2-, HgX3-, HgX42- (where X= OH-, Cl- or Br-), HgO on aerosol particles and complexes of Hg with organic acids. |
|
Nonreactive species |
CH3Hg+, CH3HgCl, CH3HgOH and other (HgCN)2, HgS and organomercurials. |
The highly toxic forms of mercury are the reactive inorganic mercury ions (Hg+2) that possess great affinity for cysteine residues of proteins and to N-atoms of nuclear material of the cell and the organomercurial monomethylmercury (CH3Hg+; MMHg) which have high affinity for tissues of central nervous system, high lipid solubility, high uptake rate across biological membranes and longer residence/removal time in biological tissues [7]. The characteristics of MMHg contribute significantly to bioaccumulation and biomagnification in mercury toxicity. Bioaccumulation is defined as the increase in the total amount of Hg in an organism over time while biomagnification of Hg (incremental increase of mercury concentration at each trophic level of the food chain) is as the increase in mercury concentrations in tissue through trophic transfer, from primary producer to terminal carnivorous consumers via food chain or food web [8]. MMHg is evenly distributed in body tissues while inorganic mercury is unevenly distributed because of its less efficiency than MMHg in crossing biological membranes [9]. Mercury uptake, distribution, and toxicity are therefore dependent on the speciation and transformations of mercury.
Methods
Literature search strategy and selection criteria
A peer-reviewed literature search was carried out by use of the key terms like “mercury toxicity”, “phytotoxicity”, “Hg-oxidation states”, “biogeochemical cycling of mercury”, “mercury detoxification systems”, “mer operon” and “met gene” from Google Scholar, Google Web Browser, PubMed Central, Springer Online Archives Collection and PubMed for this review. The retrieval of the search was done without applying any filter to limit the study type. Articles resulting from these searches and relevant references for this review were selected published from August, 1939 to December, 2014. Articles published in various languages like English, French, and German were also included. Some references are not falling in the above mentioned key terms due to their significant data or relevance to the specificity of this topic. In this comprehensive review, 72 peer reviewed research and review articles were selected.
Discussion
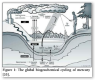
The Biogeochemical Cycle of Mercury
Mercury occurs naturally in biogeochemical system of earth, but centuries of anthropogenic activities [10] like mining and fossil fuel burning [11], have been mobilizing increasing amounts of mercury in the atmosphere [12-14], terrestrial environment [15] and aquatic systems [16-18]. Mercury can be transformed to different states by oxidation-reduction and methylation-demethylation processes. In redox reactions, the process of oxidation causes the mercury to lose electrons by changing its valency from Hg0 to Hg2+ while reduction causes the mercury to gain electrons by transforming to lower valence state [19-24].
The oxidation of mercury (Hg0) in the atmosphere is a significant phenomenon due its involvement in the dethronement of mercury in both soil and water. Metallic mercury (Hg0) can be volatilized easily into vapors and discharge to the atmosphere where they can be transmitted in air currents for a year or more and again accumulate into the environment to initiate another cycle [25,26]. In contrast, toxic mercuric state (Hg2+) can remain in the atmosphere for less than two weeks because of its low tendency to evaporate, greater solubility and reactive nature. Therefore, when elemental mercury becomes ionic mercury, it tends to quickly infuse in water as rain and snow and consequently accumulate back in the environment [27,28].
In nature, mercury changes to methylmercury upon acquisition of the methyl group (CH3). This process ends up producing extremely lethal compounds including methylmercury (MeHg +) that tends to accumulate in living cells and pass all along the food chain, from smaller organisms i.e., microbes, then to aquatic organisms i.e., fish and ultimately to humans [29-32] (Figure 1).
Uptake of Hg by Plants
Mercury toxicity has become an ever increasing challenge as a consequence of global heavy metal contamination. Considerable amount of mercury is being added to agricultural soil due to the usage of sewage sludge, chemical fertilizers, lime and manures [34-36]. The dynamic association between mercury in the soil and its absorption by the plants is not collinear and depends on many variable factors like cation-anion interchange capability, pH of soil, soil aeration and plant varieties. With the increase in pH of soil and in the vicinity of lime and salts, the mercury absorption can be decreased [37, 38].
It has been reported that the absorption of Hg is specific to the bryophyte plants, lichens, mycorrhizae, wetland plants, tracheophyte plants and crop vegetation [39]. Various aspects that affects the absorption of Hg by plants include the organic matter of soil or sediments, the interchange capability of various atoms and molecules like carbon, oxides and carbonate content, redox potential, the formulation used and the total metal content. Generally, the absorption of mercury by plants could be directly correlated to the contamination level. The absorption of Hg in most of plants is due to its accumulation in the plant roots or its absorption through shoots in the form of vapors or by translocation [40, 41]. It is considered that plants absorb the elemental form (Hg0) and accumulate it in the shoots, but not translocated to the roots [42].
Toxic mercuric ions are considered to get entry into plant cells by same recruitment mechanisms as essential nutrients compete with these ions for uptake. Mercury (Hg), categorized as class B metal prefers to bind with sulfur and nitrogenous ligands and is considered to get entry into the cell through ion channels competing with other toxic and essential metals. Nonetheless, this knowledge is primarily a result of experiments in animal cells and the authors believe that there are some other mechanisms of mercury absorption that are still under consideration [43, 44].
Phytotoxicity Induced by Hg
General Effects: The introduction of mercury in plant systems has principle importance due to its application in fertilizers, herbicides and seed disinfectants [45]. Few mercury species are being used on tree foliage as fungicides and they can be transferred, relocated and redistributed in plants.
At the cellular and subcellular level, the processes by which metals may prove lethal include obstruction of biologically significant molecules (e.g. enzymes, polynucleotides), transportation systems for micronutrients, displacement or substation of metal ions from biomolecules (such as magnesium (Mg+2) from chlorophyll), deforming and inactivating enzymatic proteins, and compromising cell membrane integrity. The possible causal processes causing Hg-induced phytotoxicity are modifications in the porosity of the outer cell envelop (cell membrane), high affinity for reactive groups like sulphydryl, phosphate, adenosine diphosphate or adenosine triphosphate, and displacement of essential ions and its capability in the disruption of several functions involving critical proteins [37, 38].
Toxic mercuric ions also disrupt the antioxidant defense mechanism by altering the modulation of non-protein thiols, non-enzymatic antioxidants glutathione, ascorbate peroxidase and glutathione reductase and the antioxidant enzyme superoxide dismutase [46-49].
The evidence of mecury phytotoxicity has been studied in various grain crops like Oryza sativa and Triticum aestivum. The primary effects of Hg compounds are on the embryo and secondary on endosperm. Hg compounds cause the breakdown of –SH- system by interfering it in biological systems resulting in the production of –S-Hg-S- bridge which may influence germination and embryo development (rich in SH ligands). In O. sativa and Zea mays, HgCl2 is involved in the obstruction of primary roots elongation as compared to shoots [37, 38].
Hg influences both light and dark reactions of photosynthesis by substituting the central atom of chlorophyll (Mg+2) by Hg in vivo which is an important damaging mechanism. It also reduces the transpiration rate, water uptake and chlorophyll synthesis. Toxic mercuric cations are involved in the loss of magnesium, potassium, manganese and deposition of iron which lead to the modifications in cell membrane porosity [50].
Genotoxicity: The cellular and molecular mechanisms that are involved in Hg-induced toxicity in plants are practically unknown due to scarce studies considering Hg genotoxicity. However, it has been shown that mercury can insert harmful genetic effects to different plant species [51].
In earlier experiments, multinucleated cells in the root tips of corn seedlings, exposed to solution of Ceresan (ethyl mercuric phosphate; a fungicide) resulted in the formation of polyploidy, aneuploidy and c-tumors through c-mitosis [52,53]. C-mitosis (colchicine treated), sister chromatid exchanges, chromosomal aberrations and spindle alterations can be stimulated by several compounds at similar dosage but butyl mercury bromide is most notable in this respect [54]. It has been reported that inorganic mercury poisoning in Allium cepa (onion) and Allium sativum (garlic) resulted in reduction of mitotic index in the cells of root tip and an increment in chromosomal aberrations that depend on concentration and time of exposure. Mercuric chloride (HgCl2) was concluded as more cytotoxic as compared to mercurous chloride (Hg2Cl2) and lowest effective concentration tested (LECT) was measured as 10 ppm. The greater tolerance of A. sativum than A. cepa was attributed to the presence of high levels of heterochromatin in the former and low amount of sulfur in the later [37, 38].
Detoxification of Hg in Soil through Microorganisms
Release of Hg from natural sources (volcanic activity and weathering of rocks) [55-57] and anthropogenic sources (fossil fuel combustions, electricity-generating grid stations, gold and mercury mining, production of chlorine, cement, caustic soda, pesticides, medical instruments, mirrors and industrial effluents etc) [58,59] poses a major menace to the soil environment [60]. Generally, heavy metals cannot be degraded by biological mechanisms and exist in the environment to an indefinite extent. After their accumulation in the soils, the lethal heavy metals adversely influence the soil microflora, including plant growth promoting rhizobacteria (PGPR) in the rhizosphere, and their physiological processes. Furthermore, the elevated concentrations of Hg and their uptake by plants also pose adverse affects on the plant growth [61], symbiotic association and ultimately the crop yields by disrupting cell organelles, and disintegrating the membranes, serving as genotoxic substance disrupting the photosynthetic and respiration processes [41,62]. Therefore, the remediation of Hg-polluted sites has become an urgent need, as these lands have covered large areas which have been interpreted inapplicable for sustainable agriculture.
Two extensively studied resistance or detoxification systems based on clustered genes on Mer operon and Met gene. The mer operons (merTPCFAD) possess variable structures and constitute a number of genes which encode various functional proteins. In Staphylococcus aureus and Bacillus sp., the merR genes which are involved in the expression of functional proteins for metalloregulation, are transcribed in that direction as the structural genes of mer operon whereas in other species, it is transcribed separately and divergently from the structural genes. merR binds the promoter-operator site of mer operon and activates/ represses the transcription of structural genes in the presence/absence of activating concentration of Hg2+ respectively [63,64]. The structural mer genes are involved in the expression of proteins which aid in the transportation like merT, merP and merC, merF and reduction (merA) of toxic form of mercury. merB genes confer resistance to many organomercurials by hydrolyzing the C-Hg bond [65]. The distal promoter gene, merD binds weakly the same operator-promoter site as and is involved in down-regulation of the mer operon [66].
The other mercury detoxification system is the expression of met gene which encodes sulfhydrylase (SHLase) enzymes. This enzyme regulates the methionine biosynthesis and results hydrogen sulfide (H2S) production. H2S reacts with toxic form of mercury (Hg+2) and precipitate it into nontoxic mercuric sulfide (HgS) [67-69]. Detoxification mechanisms that employ different microbes to take off environmental contaminants have obtained a profound interest in the recent years [70]. The commonly used bacterial and yeast genera in the bioremediation of Hg include Bacillus, Pseudomonas, Citrobacter, Klebsiella, Rhodobacter and Saccharomyces, Candida and Pichia respectively [37, 38, 71, 72].
Thus, by applying these microorganisms as a biofertilizers to Hg-contaminated soils, the toxicity of Hg can be reduced resulting in the enhancement of soil fertility and crop productivity which aids in sustainable agriculture.
Conclusion
In conclusion, mercury is a hazardous contaminant associated with serious problems in plants and animals because it can be easily spread through many ecosystems. Unfortunately, very less information is available about phytotoxicity caused by Hg, processes by which Hg is absorbed by plant cells and detoxification mechanisms by which it is modified from toxic to nontoxic form in soil through microorganisms. Although plants attribute a significant role as the base of several trophic levels in food chain particularly of humankind subsistence and thriftiness, therefore, it is an urgent necessity to step up the knowledge about the mechanisms of Hg uptake by plants, its phytotoxicity and detoxification mechanisms of this pollutant. The mini review presented here will provide a worthy rootage for other scientists engaged to research on Hg-induced phytotoxicity and its modification or detoxification processes to stimulate foster research in this field.
References
- Weast R. Handbook of chemistry and physics: CRC Press, Cleveland, OH. (1973):
- Bunn WB, Mcgill CM, Barber TE, Cromer JW, Goldwater LJ. Mercury exposure in chloralkali plants. American Industrial Hygiene Association Journal, (1986); 47 (5): 249-254.
- Goldwater LJ. Mercury in dentistry. Journal of Toxicology Clinical Toxicology, (1991); 29 (2): 151-164.
- Antonovich VP, Bezlutskaya IV. Speciation of mercury in environmental samples. Journal of Analytical Chemistry, (1996); 51 (1): 106-113.
- Agocs M, Clarkson T, Ambre J, Becker C, Borak J, et al. Mercury toxicity. American Family Physician, (1992); 46 (6): 1731-1741.
- Joshi D, Mittal DK, Shukla S. Mercury toxicity – A global problem-protection by combination therapy – Novel approach. Toxicology Letters, (2011); 205: S127-S127.
- Baldi F. Microbial transformation of mercury species and their importance in the biogeochemical cycle of mercury. Metal Ions in Biological Systems, (1997); 34: 213-257.
- Boudou A, Ribeyre F. Mercury in the food web: Accumulation and transfer mechanisms. Metal Ions in Biological Systems, (1997); 34: 289-319.
- Meili M. Mercury in lakes and rivers. Metal Ions in Biological Systems, (1997); 34: 21-51.
- Kim KH, Shon ZH, Nguyen HT, Jung K, Park CG, et al. The effect of man made source processes on the behavior of total gaseous mercury in air: A comparison between four urban monitoring sites in Seoul Korea. Science of the Total Environment, (2011); 409 (19): 3801-3811.
- Kostova I, Vassileva C, Hower J, Mastalerz M, Vassilev S, et al. Mercury in coals and fly ashes from Republika and Bobov Dol thermoelectric power plants. Comptes Rendus De L Académie Bulgare Des Sciences, (2011); 64 (2): 253-262.
- Gustin MS, Taylor GE, Leonard TL. Atmospheric mercury concentrations above mercury contaminated mill tailings in the Carson River Drainage-Basin, Nv. Water Air and Soil Pollution, (1995); 80 (1-4): 217-220.
- Gosar M, Pirc S, Sajn R, Bidovec M, Mashyanov NR, et al. Distribution of mercury in the atmosphere over Idrija, Slovenia. Environmental Geochemistry and Health, (1997); 19 (3): 101-110.
- Aspmo K, Temme C, Berg T, Ferrari C, Gauchard LP, et al. Mercury in the atmosphere, snow and melt water ponds in the North Atlantic Ocean during Arctic summer. Environmental Science and Technology, (2006); 40 (13): 4083-4089.
- Bargagli R, Agnorelli C, Borghini F, Monaci F. Enhanced deposition and bioaccumulation of mercury in Antarctic terrestrial ecosystems facing a coastal polynya. Environmental Science and Technology, (2005); 39 (21): 8150-8155.
- Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, et al. The movement of aquatic mercury through terrestrial food webs. Science, (2008); 320 (5874): 335.
- Havlik B, Stary J, Prasilova J, Kratzer K, Hanusova J. Mercury circulation in aquatic environment .2. Metabolism of methyl and phenyl mercury in phytoplankton. Acta Hydrochimica et Hydrobiologica, (1979); 7 (4): 401-408.
- Kusik BW, Carvan MJ, Udvadia AJ. Detection of mercury in aquatic environments using EPRE Reporter Zebrafish. Marine Biotechnology, (2008); 10 (6): 750-757.
- Byun Y, Ko KB, Cho M, Namkung W, Shin DN, et al. Oxidation of elemental mercury using atmospheric pressure non-thermal plasma. Chemosphere, (2008); 72 (4): 652-658.
- Pedersen GC. Mercury chemistry. Journal of the Air and Waste Management Association, (2001); 51 (10): 1390.
- Presto AA, Granite EJ. Survey of catalysts for oxidation of mercury in flue gas. Environmental Science and Technology, (2006); 40 (18): 5601-5609.
- Smith T, Pitts K, McGarvey JA, Summers AO. Bacterial oxidation of mercury metal vapor, Hg(0). Applied and Environmental Microbiology, (1998); 64 (4): 1328-1332.
- Si L, Ariya PA. Aqueous photoreduction of oxidized mercury species in presence of selected alkanethiols. Chemosphere, (2011); 84 (8): 1079-1084.
- Zheng W, Liang L, Gu B. Mercury reduction and oxidation by reduced natural organic matter in anoxic environments. Environmental Science and Technology, (2012); 46 (1): 292-299.
- Araujo RGO, Vignola F, Castilho INB, Borges DLG, Welz B, et al. Determination of mercury in airborne particulate matter collected on glass fiber filters using high-resolution continuum source graphite furnace atomic absorption spectrometry and direct solid sampling. Spectrochimica Acta Part B-Atomic Spectroscopy, (2011); 66 (5): 378-382.
- Wangberg I, Munthe J, Amouroux D, Andersson ME, Fajon V, et al. Atmospheric mercury at mediterranean coastal stations. Environmental Fluid Mechanics, (2008); 8 (2): 101-116.
- Landing WM, Guentzel JL, Gill GA, Pollman CD. Methods for measuring mercury in rainfall and aerosols in Florida. Atmospheric Environment, (1998); 32 (5): 909-918.
- Kieber RJ, Parler NE, Skrabal SA, Willey JD. Speciation and photochemistry of mercury in rainwater. Journal of Atmospheric Chemistry, (2008); 60 (2): 153-168.
- Shepotko AO, Lomonosov IS, Karpov IK. Physicochemical conditions of mercury methylation in water reservoirs. Doklady Akademii Nauk SSSR, (1990); 311 (1): 204-207.
- Clarkson TW. Human toxicology of mercury. Journal of Trace Elements in Experimental Medicine, (1998); 11 (2-3): 303-317.
- Zhu H, Yan BX, Cao HC, Wang LX. Risk assessment for methylmercury in fish from the Songhua River, China: 30 years after mercury-containing wastewater outfalls were eliminated. Environmental Monitoring and Assessment, (2012); 184 (1): 77-88.
- Monson BA, Brezonik PL. Seasonal patterns of mercury species in water and plankton from softwater lakes in Northeastern Minnesota. Biogeochemistry, (1998); 40 (2-3): 147-162.
- University of Wisconsin-Eau Claire (2014) Mercury in the Environment and Water Supply.url: https://people.uwec.edu/piercech/Hg/mercury_water/cycling.htm. (Last accessed on 2 December 2014)
- Zheng YM, Liu YR, Hu HQ, He JZ. Mercury in soils of three agricultural experimental stations with long-term fertilization in China. Chemosphere, (2008); 72 (9): 1274-1278.
- Zhao XL, Wang DY. Mercury in some chemical fertilizers and the effect of calcium superphosphate on mercury uptake by corn seedlings (Zea mays L.). Journal of Environmental Sciences (China), (2010); 22 (8): 1184-1188.
- Cappon CJ. Content and chemical form of mercury and selenium in soil, sludge, and fertilizer materials. Water Air and Soil Pollution, (1984); 22 (1): 95-104.
- Patra M, Bhowmik N, Bandopadhyay B, Sharma A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environmental and Experimental Botany, (2004); 52 (3): 199-223.
- Patra M, Sharma A. Mercury toxicity in plants. Botanical Review, (2000); 66 (3): 379-422.
- Hussein HS, Ruiz ON, Terry N, Daniell H. Phytoremediation of mercury and organomercurials in chloroplast transgenic plants: enhanced root uptake, translocation to shoots, and volatilization. Environmental Science and Technology, (2007); 41 (24): 8439-8446.
- Tomiyasu T, Matsuo T, Miyamoto J, Imura R, Anazawa K, et al. Low level mercury uptake by plants from natural environments-mercury distribution in Solidago altissima L. Environmental Sciences, (2005); 12 (4): 231-238.
- Perez-Sanz A, Millan R, Sierra MJ, Alarcon R, Garcia P, et al. Mercury uptake by Silene vulgaris grown on contaminated spiked soils. Journal of Environmental Management, (2012); 95: S233-S237.
- Suszcynsky EM, Shann JR. Phytotoxicity and accumulation of mercury in Tobacco subjected to different exposure routes. Environmental Toxicology and Chemistry, (1995); 14 (1): 61-67.
- Blazka ME, Shaikh ZA. Cadmium and mercury accumulation in rat hepatocytes: interactions with other metal ions. Toxicology and Applied Pharmacology, (1992); 113 (1): 118-125.
- Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemistry Letters, (2010); 8 (3): 199-216.
- Cavallini A, Natali L, Durante M, Maserti B. Mercury uptake, distribution and DNA affinity in durum wheat (Triticum durum Desf.) plants. Science of the Total Environment, (1999); 244: 119-127.
- Sparks DL. Toxic metals in the environment: The role of surfaces. Elements, (2005); 1 (4): 193-197.
- Ortega-Villasante C, Rellan-Alvarez R, Del Campo FF, Carpena-Ruiz RO, Hernandez LE. Cellular damage induced by cadmium and mercury in Medicago sativa. Journal of Experimental Botany, (2005); 56 (418): 2239-2251.
- Israr M, Sahi S, Datta R, Sarkar D. Bioaccumulation and physiological effects of mercury in Sesbania drummondii. Chemosphere, (2006); 65 (4): 591-598.
- Calgaroto NS, Castro GY, Cargnelutti D, Pereira LB, Goncalves JF, et al. Antioxidant system activation by mercury in Pfaffia glomerata plantlets. Biometals, (2010); 23 (2): 295-305.
- Boening DW. Ecological effects, transport, and fate of mercury: a general review. Chemosphere, (2000); 40 (12): 1335-1351.
- De Flora S, Bennicelli C, Bagnasco M. Genotoxicity of mercury compounds. A review. Mutation Research, (1994); 317 (1): 57-79.
- Kostoff D. Effect of the fungicide “Granosan” on atypical growth and chromosome doubling in plants. Nature, (1939); 144: 334.
- Kostoff D. Atypical growth, abnormal mitosis and polyploidy induced by ethyl-mercury-chloride. Journal of Phytopathology (Berlin), (1940); 13: 91-96.
- FiskesjÖ G. Some results from allium tests with organic mercury halogenides. Hereditas, (1969); 62 (3): 314-322.
- Baeyens W, Dehandschutter B, Leermakers M, Bobrov VA, Hus R, et al. Natural mercury levels in geological enriched and geological active areas: Case study of Katun river and Lake Teletskoye, Altai (Siberia). Water Air and Soil Pollution, (2003); 142 (1-4): 375-393.
- Ando T, Yamamoto M, Tomiyasu T, Tsuji M, Akiba S. Mercury distribution in seawater of Kagoshima Bay near the active volcano, Mt. Sakurajima in Japan. Bulletin of Environmental Contamination and Toxicology, (2010); 84 (4): 477-481.
- Bagnato E, Aiuppa A, Parello F, Allard P, Shinohara H, et al. New clues on the contribution of Earth's volcanism to the global mercury cycle. Bulletin of Volcanology, (2011); 73 (5): 497-510.
- Fu XW, Feng XB, Zhang H, Yu B, Chen LG. Mercury emissions from natural surfaces highly impacted by human activities in Guangzhou province, South China. Atmospheric Environment, (2012); 54: 185-193.
- Dabrowski JM, Ashton PJ, Murray K, Leaner JJ, Mason RP. Anthropogenic mercury emissions in South Africa: Coal combustion in power plants. Atmospheric Environment, (2008); 42 (27): 6620-6626.
- de Oliveira SMB, Melfi AJ, Fostier AH, Forti MC, Favaro DIT, et al. Soils as an important sink for mercury in the Amazon. Water Air and Soil Pollution, (2001); 126 (3-4): 321-337.
- Han FX, Su Y, Monts DL, Waggoner CA, Plodinec MJ. Binding, distribution, and plant uptake of mercury in a soil from Oak Ridge, Tennessee, USA. Science of the Total Environment, (2006); 368 (2-3): 753-768.
- Piehler M, Swistak J, Pinckney J, Paerl H. Stimulation of diesel fuel biodegradation by indigenous nitrogen fixing bacterial consortia. Microbial Ecology, (1999); 38 (1): 69-78.
- Wang Y, Moore M, Levinson H, Silver S, Walsh C, et al. Nucleotide sequence of a chromosomal mercury resistance determinant from a Bacillus sp. with broad-spectrum mercury resistance. Journal of Bacteriology, (1989); 171 (1): 83-92.
- Laddaga RA, Chu L, Misra T, Silver S. Nucleotide sequence and expression of the mercurial-resistance operon from Staphylococcus aureus plasmid pI258. Proceedings of the National Academy of Sciences, (1987); 84 (15): 5106-5110.
- Osborn AM, Bruce KD, Strike P, Ritchie DA. Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiology Reviews, (1997); 19 (4): 239-262.
- Mukhopadhyay D, Yu H, Nucifora G, Misra T. Purification and functional characterization of MerD. A coregulator of the mercury resistance operon in gram-negative bacteria. Journal of Biological Chemistry, (1991); 266 (28): 18538-18542.
- Ray S, Gachhui R, Pahan K, Chaudhury J, Mandal A. Detoxification of mercury and organomercurials by nitrogen-fixing soil bacteria. Journal of Biosciences, (1989); 14 (2): 173-182.
- Ono B, Ishii N, Fujino S, Aoyama I. Role of hydrosulfide ions (HS-) in methylmercury resistance in Saccharomyces cerevisiae. Applied and Environmental Microbiology, (1991); 57 (11): 3183-3186.
- Ono B, Kijima K, Ishii N, Kawato T, Matsuda A, et al. Regulation of sulphate assimilation in Saccharomyces cerevisiae. Yeast, (1996); 12 (11): 1153-1162.
- Gupta N, Ali A. Mercury volatilization by R factor systems in Escherichia coli isolated from aquatic environments of India. Current Microbiology, (2004); 48 (2): 88-96.
- Amin A, Latif Z. Isolation and characterization of H2S producing yeast to detoxify mercury containing compounds. International Research Journal of Microbiology, (2011); 2: 517-525.
- Amin A, Latif Z. Detoxification of mercury pollutant by immobilized yeast strain Candida xylopsoci. Pakistan Journal of Botany, (2013); 45 (4): 1437-1442.