Short Communication
Qualitative Phytochemical Analysis and Microbial Inhibitory Activities of Pacific Rain Tree (Samanea saman (Jacq.) Merr.) Pods
James Kennard S. Jacob*1, Eden S. David 2
Adv. life sci., vol. 3, no. 4, pp. 125-129, August 2016
*- Corresponding Author: James Kennard S. Jacob (Email: james.jacobmsc@gmail.com)
Authors' Affiliations
2- Faculty, Department of Biological Sciences, College of Arts and Sciences, Central Luzon State University, Science City of Munoz, Nueva Ecija 3120, Philippines
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Crop diseases and human health are always at stake and the emerging problem on the use of synthetic anti-pathogens and medicine is one of the most difficult to combat. The first step towards determining such capabilities among plants is to determine their phytochemicals.
Methods: Eight preliminary phytochemical tests was done on Samanea saman which includes, test for alkaloids saponins, flavonoids, tannins, glycosides, steroids, terpenoids and resins. Powdered pods were subjected to ethanol and aqueous extraction. Extracts were also tested for its antifungal and anti-microbial properties against Fusarium oxysporum, E. coli and S. aureus, respectively.
Results: Out of the eight phytochemical tests done, seven (7) were found to be present both on the ethanol and aqueous extracts namely, alkaloids, saponins, tannins, glycosides, steroids, terpenoids and resins. However, flavonoids is absent. The statistical results exhibited that there is a significant difference on the inhibitory effects against in-vitro bioassay of Fusarium oxysporum which is known to cause crop wilts and the two bacterial pathogens E. coli and S. aureus.
Conclusions: The presence of such phytochemicals in Samanea saman pods revealed that it can be a basis of new, natural and non-synthetic treatments. This finding suggests that its pods can be used as antibacterial and antifungal source.
Key words: Bioassay, Crop wilts, Disc diffusion, Extracts, Phytochemicals
Introduction
For years, the use of antipathogens such as antibacterial and antifungal synthetic drugs to control crop disease has contributed to increased production of food worldwide. Plant diseases are caused by many pathogens such as fungi, bacteria, nematodes and viruses. Among the list of many pathogens affecting plants, fungi is the major contributor causing about 90% yield loss and most of the agricultural plants have been reported to have at least one type of Fusarium associated disease, a destructive disease that has led to significant yield and quality losses for farmers and to contamination of its mycotoxins [1]. Several effective synthetic anti-pathogenic drugs are now being studied for their effects of on human health and environment and many research efforts have been carried out to find alternatives and environmentally safe methods that can be used to control plant diseases [2]. With the increasing pesticide residues concern in agricultural products and environment as well as the incidence of pathogen-resistant chemical pesticides, the use of non-chemical based and eco-friendly methods that includes natural metabolites have assumed greater significance for a better crop yield [3]. To counteract with the highlighted challenge, we must develop a potent anti-pathogens against the already emerged resistant pathogen strains and even the emerging ones [4].
In the Philippines, Samanea saman, also known as Akasya (Filipino), Rain Tree or Monkey Pod (English) belonging to family Fabaceae, is easily recognized by its umbrella-shaped canopy that usually reaches 15-25m (50-80ft) in height. It is an important tree in the Pacific as a shade tree on small farms, along roads, in parks and pastures [5]. Many plants possess antimicrobial activities and are used for the treatment of different diseases dated back to prehistoric tradition which uses natural substances or extracts that possesses broad spectrum of synthetic activity and have been the source of many useful compounds [6,7]. With these findings, an emerging interest in the possible application of these phytochemicals in the development of new drugs for human and plant disease management is at stake. In addition, the knowledge shifted the direction for new drug search towards plant sources thereby leading to the recent increased studies on different solvent extracts of plant species originally used in traditional practice [1,8,9].
Methods
Collection and Extraction of Samples
The pods used in this study were collected from the trees surrounding the college from April to May. The pods were sundried for 48 hours prior storage on a Ziploc for storage. Pods used were oven dried for six (6) hours at 70ºC and powderized using mortar and pestle separating the seeds. For the preparation of ethanol extracts, 50g of powdered S. saman pods were soaked in 250ml 95% ethanol for 48 hours at room temperature. The suspension was filtered and filtrate was subjected to a rotary evaporator for 200rpm. For the preparation of aqueous extracts, 50g of powdered S. saman pods were suspended in a 250ml sterilized distilled water for 24 hours. Suspension was filtered using wattman’s filter paper no. 01 and filtrate was stored in a sterile amber bottles and kept refrigerated prior use.
Phytochemical Screening Test
Biochemical tests for the screening and identification of bioactive chemical constituents in the medicinal constituents under study were carried out in extracts using the standard procedures as described by [7,10].
Test for alkaloids
Extracts were dissolved individually in diluted hydrochloric acid and filtered. Filtrates were treated with Wagner’s reagent (solution of iodine in potassium iodide). Formation of a reddish brown colored precipitate indicated the presence of alkaloids.
Test for Saponins
To test the saponins, 2g of powdered sample was boiled together with 20ml of distilled water in a water bath and filtered. 10ml of the filtered sample was mixed with 5ml of distilled water in a test tube and shaken vigorously to obtain a stable persistent froth. The frothing was then mixed with 3 drops of olive oil and for the formation of emulsion which indicated the presence of saponins.
Test for Flavonoids
To determine the presence of flavonoids, 2 to 3 drops of 1% NH3 solution was added to the aqueous extract of each sample in a test tube. A yellow coloration is observed if flavonoid compound was present.
Test for Tannins
Tannins were determined by boiling 0.5g of powdered sample in a 20ml distilled water in a test tube and filtered. 0.1% FeCl3 was added to the filtered samples and observed for brownish to green or a blue to black coloration which showed the presence of tannins. Green coloration indicated the presence of gallotannins while brown coloration indicated the presence of pseudotannins.
Glycosides
Test for glycosides was determined by preparing 1ml of concentrated H2SO4 in a test tube where 5 ml of aqueous extract from the sample was mixed with 2mL of glacial acetic acid containing 1 drop of FeCl3. The above mixture was carefully added to 1ml of concentrated H2SO4 so that it is underneath the mixture. In presence of cardiac glycoside in the sample, a brown ring would appear indicating the availability of the cardiac glycoside constituent.
Evaluation of the Microbial Inhibitory Activities of Rain Tree Pods
Dilution of Extracts
Each extracts were diluted on the following concentrations: for the antibacterial assay, 10ppm, 100ppm and 1000ppm was used and for the antifungal assay, 1mg/ml, 5mg/ml and 10mg/ml was used.
Source of Microbial Cultures
Pure cultures of Fusarium oxysporum was obtained from the Laboratory of fungal collection of RM-CARES, Research and Extension, CLSU. Bacterial strains, E. coli and S. aureus was obtained from the bacterial culture collection of Department of Biological Sciences, College of Arts and Sciences, CLSU.
Antifungal Assay
Antifungal assay was carried out using 2ml of prepared ethanol extracts at different concentrations were aseptically poured on a sterile standard plate and approximately 15-20ml of sterilized Potato Dextrose Agar (PDA) was added following standard microbiological protocols. Every plate containing mixtures were aseptically inoculated with a 10-mm fungal disk. Inoculated standard plates were incubated on a room temperature (28-32°C) and growth was measured using calibrated Vernier caliper for every 24-hours for 5 days.
Antibacterial Assay
The antibacterial assay was done following the Kirby-Bauer Method against E.coli and S. aureus. Six (6) mm of sterile paper discs were soaked on the ethanol and aqueous extracts for about 30-40 mins to allow absorption of the extracts. The discs were aseptically inoculated onto the plates with the test organisms with proper labels each disc. Zones of inhibition was observed at 8th, 16th and 24th hour of incubation.
Statistical Analysis
Analysis was laid out Completely Randomized Design (CRD) with three (3) replications per treatment combination. The results presented are the means ± standard deviation of three replicates. The recorded data were treated statistically using the one way analysis of variance (ANOVA). The means were compared by Least Significant Difference test at p < 0.05 using SPSS v.20.
Results
Phytochemical Analysis
Phytochemicals are naturally occurring constituents of plants. Researches have been widened to prove the so-called bioactivities of these constituents. The presence of these phytochemical constituents were carried out in this present study. Samanea saman pods exhibited low to high presence of the different biochemical constituents present. Alkaloids present on both the ethanol and aqueous extract are moderate in amount. On the other hand, results exuded by ethanol extracts on the presence of saponins was greatly higher than aqueous extracts (Table 1). However, flavonoids was absent on both ethanol and aqueous extract. Moreover, tannins, glycosides, steroids, terpenoids and resins on ethanol extracts were present in moderate appreciable amounts while on aqueous extracts only glycosides and resins showed moderate presence but significantly lower on the presence of steroids and terpenoids.
Anti-fungal Bioassay
After 5 days of incubation, it was observed evidently that ethanol extracts at 10mg/ml had exhibited a mean diameter of 39.51mm which is significantly lower then the results showed by the ethanol extracts having 1mg/ml (53.85mm) and 5mg/ml (48.05mm) against the control with 56.93mm (Table 2). The observation on the aqueous extracts at different levels of concentration also indicated that at 10mg/ml, the growth of F. oxysporum can be stunted at 41.96mm compared to the results of 1mg/ml (48.96mm) which is comparable to the results of the control (48.98mm) and 5mg/ml (46.69mm).
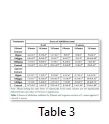
Antibacterial Bioassay
The extracts of S. saman (Table 3) were found to have inhibitory effects on the gram negative bacteria E. coli and the gram positive bacteria S. aureus. After 24 hours of observation it was found out that at 1000ppm ethanol extracts of S. saman against E. coli and S. aureus inhibited 13.35mm and 11.10mm respectively, significantly higher inhibition compared to the results exuded by 10ppm (11.11mm, 9.55mm) and 100ppm (12.11mm, 10.43mm). The same trend was observed on the aqueous extracts against E. coli and S.aureus. At 1000ppm, the extracts inhibited 9.42mm and 9.36mm, statistically higher inhibition than 10ppm (9.03mm and 9.36mm) and 100ppm (10.38mm and 10.18mm).
Tables & Figures
Discussion
Biochemical constituents plays a significant role in human health. For instance, the presence of alkaloids in perceptible amounts has been testified to act as a pain reliever and a contemporary anaesthetic in ophthalmology with stimulating result and antipyretic effects as other functions [11]. As reported by Fenwick et al., [12], the presence of saponins includes major biological effects such as erythrocyte hemolysis, enzyme inhibition, cholesterol and bile acid metabolism, antifungal activity, anti-carcinogenic and effect on the reproduction which is evidently present on Samanea saman pods. Biochemical constituents present in the ethanol and aqueous extracts showed inhibitory effects on both antifungal and antibacterial bioassays. Tannins are known antimicrobial agents that could inhibit the growth of microorganisms by precipitating out the microbial protein and thus depriving them of nutritional proteins needed for their growth and development [13]. The presence of tannins in plants can cause negative effect on productivity, reduced nutrient availability, reduced digestibility, impaired digestive physiology and may be mucosal perturbations for those who will intake such plants. While the occurrence of terpenoids in plants could cause cytotoxic effects, growth hormones and tumor promoters and plants containing alkaloids have high nitrogen organic constituents which can be attributed to their ability to become poisonous and even addictive [14]. The results of this present study apparently highlighted the scientific foundation for the possible use of this plant in an ethno-medication and the probable intervening effectiveness of ethanol and aqueous extracts. Thus, the ethanol and aqueous extracts of S. saman pods appeared to be a better source of natural but narrow spectrum antimicrobial. In conclusion, the phytochemical components and antimicrobial activity results of the present study suggests that S. saman pods could serve as a good source for foods, raw materials, and biodiesel industries. This also suggested that the therapeutic potency of S. saman may be dependent on the extraction solvent used and it is strongly suggested that other extraction method be used in which our laboratory in currently aligned, and finally the extraction and characterization of the detected phytochemicals in the S. saman pods might result in the elucidation of its active therapeutic compound.
References
- Rongai D, Milano F, Sciò E. Inhibitory effect of plant extracts on conidial germination of the phytopathogenic fungus Fusarium oxysporum. American Journal of Plant Sciences, (2012); 3(12): 1693.
- Hanaa RF, Abdou ZA, Salama DA, Ibrahim MA, Sror H. Effect of neem and willow aqueous extracts on Fusarium wilt disease in tomato seedlings: Induction of antioxidant defensive enzymes. Annals of Agricultural Sciences, (2011); 56(1): 1-7.s
- Howell C. Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Disease, (2003); 87(1): 4-10.
- Obasi Nnamdi L, Egbuonu A, Ukoha P, Ejikeme P. Comparative phytochemical and antimicrobial screening of some solvent extracts of Samanea saman (fabaceae or mimosaceae) pods. African Journal of Pure and Applied Chemistry, (2010); 4206-212.
- Durr P. The biology, ecology and agroforestry potential of the raintree, Samanea saman (Jacq.) Merr. Agroforestry Systems, (2001); 51(3): 223-237.
- Zasloff M. Antimicrobial peptides of multicellular organisms. nature, (2002); 415(6870): 389-395.
- Sofowora A. Recent trends in research into African medicinal plants. Journal of Ethnopharmacology, (1993); 38(2-3): 197-208.
- Rios J, Recio M. Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology, (2005); 100(1): 80-84.
- Dwivedi S. Efficacy of some Medicinal Plant Extract Against Fusarium oxysporum f. sp. ciceri Causing Chickpea Wilt. Asian Journal of Crop Science, (2015); 7(2): 138.
- Ghaima KK, Hashim NM, Ali SA. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale). Journal of Applied Pharmaceutical Science, (2013); 3(5): 96.
- Yadav R, Agarwala M. Phytochemical analysis of some medicinal plants. Journal of Phytology, (2011); 3(12).
- Fenwick DE, Oakenfull D. Saponin content of food plants and some prepared foods. Journal of the Science of Food and Agriculture, (1983); 34(2): 186-191.
- Mann A, Banso A, Clifford L. An antifungal property of crude plant extracts from Anogeissus leiocarpus and Terminalia avicennioides. Tanzania Journal of Health Research, (2008); 10(1): 34-38.
- Jacob JKS, David ES, Undan JR. Fungal inhibiting capacity of an ethnobotanical plant from Imugan, Nueva Vizcaya against Fusarium oxysporum and Fusarium moniliforme. International Journal of Agricultural Technology, (2016); 12(1): 167-171.