Short Communication
Phytochemical and biological screening of Berberis aristata
Muhammad Rizwan1, Alamgir Khan1, Hira Nasir2, Aslam Javed3, Syed Zawar Shah1*
Adv. life sci., vol. 5, no. 1, pp. 01-07, November 2017
*- Corresponding Author: Syed Zawar Shah (Email: syed.zawar01@gmail.com)
Authors' Affiliations
2. Department of Biotechnology, Kinnaird College for Women – Pakistan
3. Center of Biotechnology and Microbiology, University of Peshawar , Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Berberis aristata occupies significant position as a medicinal plant. Given its clinical applications and the grave concern of weed based crop damage in Pakistan, the plant was investigated for its antimicrobial and allelopathic activities.
Methods: Fresh Berberis aristata plant was obtained from Rawalakot and Hajeera (District Poonch) Azad Kashmir. Methanolic extract preparation and phytochemical analysis was done using standard procedures. Antibacterial and antifungal activities of the root, stem and leaf extracts of the plant were assayed against the bacterial strains E. coli, S. typhi, S. aureus, Shigella, Citrobacter, P. vulgaris, Enterobacter, S. pyrogenes, V. cholera and Klebsiella spp. and fungal strains A. niger, Cladosporium, Rhizoctonia, Alternaria, Trichoderma, Penicillium, Curvularia, Paecilomyces and Rhizopus using disc diffusion method. Also, the phytoxicity of the extracts was evaluated against Lemna minor and the data was recorded after seven days.
Results: Phytochemical screening of the three extracts identified the presence of alkaloids, reducing sugars, steroids, flavonoids, terpenoids, glycosides and saponins while tannins were found to be absent. The leaf extract also showed negative tests for alkaloids and steroids. The extracts significantly inhibited the growth of the employed microbial isolates. The leaf extract, however, was not active against A. niger, Curvularia, Paecilomyces and Rhizopus. For most of the tested strains, the effectiveness of the extracts was much higher than that of Amoxicillin and Fluconazole; the positive controls used for bacterial and fungal cultures, respectively. All the extracts demonstrated 100% phytotoxicity against Lemna minor at 1000 μg/mL while low activity (10-20%) was observed at 10 μg/mL and 100 μg/mL, respectively.
Conclusion: The results strongly support the profound ethnobotanical applications of this plant and also demonstrate its potential for use in weed control strategies.
Key words: Cell cycle, CDK2, Cancer, Phytochemicals, Molecular docking
Introduction
Medicinal plants are a rich and natural source of bioactive constituents which have important role in modern medicine [1]. Medicinal flora is being extensively investigated for the development of new drugs that can treat microbial infections cheaply and with a good safety profile as compared to the allopathic medicines that are associated with undesirable side effects [2]. Also, with the increasing global threat of microbial antibiotic resistance, the scientists face the challenge to discover novel and safe sources of antimicrobial drugs against pathogenic microorganisms [3]. Medicinal plants offer immense opportunities for different biological screenings owing to their widespread prevalence, easy access and use in folklore medicine [4]. At present, medicinal biologists are seeking much interest in the pharmacological exploration of natural products [5] which would inevitably power the development of a greener pharmacy and contribute significantly to biomedicine. In fact, a considerable number of antibiotics available in the market have natural or semi-synthetic origins [6] and act substantially against human pathogens.
Berberis aristata, belonging to the family Berberidaceae, is a medicinal plant that is native to Himalayas in Nepal and in India. In Pakistan, it is mainly present in Azad Jammu and Kashmir and Hazara Division (KPK). It is commonly known as daruharidra and locally named as Sumbal in Azad Jammu and Kashmir. It is a large deciduous shrub, usually 1.7–3.5 meters in height. The plant has glossy dark green and obovate leaves, stalked flowers and woody, yellowish brown roots with a thin covering of bark [7].
The roots, stems, leaves and fruits of Berberis aristata are traditionally used to treat wounds, diabetes, inflammations and jaundice [8]. The extracts of this plant have reported antibacterial, antiviral, antifungal, anti-cancer, anti-inflammatory and antidiabetic profiles [9-12]. It has been used in folklore medicine against ocular trachoma infections, diarrhea and intestinal parasitic infections [10]. The most important constituent of the plant is berberine, a quaternary isoquinoline alkaloid that is typically found in the roots and stems. Various clinical trials on this alkaloid have established its therapeutic effects against neurological and cardiovascular disorders [13-15]. The root extract of B. aristata is employed as an anti-periodic, diaphoretic and antipyretic, and its action was believed to be as powerful as quinine [11]. Some preliminary reports have described the anticancer activity of methanolic extracts of B. aristata against human hepatoma cells, mouse leukemic L1210 cells and colon cancer cells which may be attributed to its COX-II inhibitory property [16,17]. The plant extracts also demonstrate significant antioxidant potential [18]. The aqueous extract of dried leaves of B. aristata showed remarkable antidysentric and antidiarrheal activity in animals [19].
In Pakistan, each year there are enormous reports of spoilage of cereal crops due to poor weed control. The weed based damage is typically more pronounced than that due to plant pests and diseases, but its effects are usually unseen and thus ignored [20]. The weeds reduce crop yield owing to their mutual antagonism for minerals, water and sunlight. Also, they provide habitat for insects that can cause crop spoilage by spreading various diseases. The synthetic herbicides impose serious environmental problems which, ultimately, afflict human health. Thus, the scientists are challenged to establish methods for controlling the weeds, with little or no side effects, to save the crop yields. Lemna bioassay technique is useful for probing natural herbicides [21] and different medicinal plants have been shown to exhibit excellent phytotoxicity [22-24]. Based on these premises, the current study aims to investigate the presence of phytochemicals and evaluate the antibacterial, antifungal and phytotoxic potential of the leaves, stem and root extracts of Berberis aristata.
Methods
Plant Material
Fresh Berberis aristata plants were procured from Rawalakot and Hajeera (District Poonch) Azad Kashmir in January, 2015. Taxonomic authentication of the plant specimen was confirmed in Department of Botany, University of Peshawar, Pakistan.
Sample Preparation
The plant was cleaned off dirt and unwanted materials by successively washing with running tap water and sterile water for 2-3 times. Different plant parts i.e. leaves, stems and roots were carefully separated and spread on clean surfaces. These were then dried under a shade for consecutive 3-4 weeks at room temperature under surveillance to avoid any microbial contamination and to provide protection from the effects of humidity [25]. The dried plant parts were then separately ground into fine powder using an electrical grinder.
Crude Extraction
100 grams of each of dried and powdered plant part was respectively extracted with methanol in specially designed containers. Each sample was dipped well in the solvent, shaken to mix and macerated in the desired container for 24 hours at room temperature [26]. The containers were kept closed to prevent evaporation. After soaking, the supernatant solvent was collected by coarse cloth filtration and the solvents were subjected to evaporation on a water bath at 40-60°C. The resulting concentrated crude extracts were used for phytochemical and biological assays [27].
Phytochemical Analysis
Qualitative phytochemical analysis of the methanolic extracts of B.aristata was done to screen the presence of phytochemical constituents like terpenoids, saponins, flavonoids, glycosides, tannins, alkaloids, reducing sugars and steroids by following the standard protocols [27].
Test for Alkaloids
Mayer’s test was performed by gently heating 0.2g of extract with 2% H2SO4 in a test tube for about two minutes. The filtrate was taken and addition of four drops of Dragendorf reagent yielded an orange red precipitate that confirmed the presence of alkaloids [27].
Test for Tannins
Ferric chloride test was performed in a test tube by mixing 2g of plant extract with 2 drops of 5% ferric chloride solution. A dark green coloration was indicative of the presence of tannins [27].
Test for Steroids
1g of the extract was taken in a test tube to which addition of 2 mL of acetic acid, 4 drops of chloroform followed by 2 drops of concentrated sulphuric acid was successively done. The formation of a reddish brown ring at the interface suggested the presence of steroids [27].
Test for Glycosides
Glycosides were tested by dissolving a small amount of extract in 1 mL of water. 5% sodium hydroxide solution was then added to the mixture; yellow coloration was indicative of the presence of glycosides.
Test for Saponins
Frothing test was performed by mixing 0.2g of the extract with 1 ml of distilled water. The mixture was heated to boil. Formation of a small frothy mass of bubbles implied the presence of saponins.
Test for Flavonoids
In a test tube, 2g of the extract was taken to which 10 mL of DMSO and metal (lead) were added. 6 drops of concentrated hydrochloric acid were then carefully added. The mixture was heated and the resulting red color signified the presence of flavonoids [28].
Test for Reducing Sugars
Fehling’s test was performed by adding a few drops of Fehling’s solution A and B to 10 mL of extract; a brick red precipitate indicated the presence of reducing sugars [17].
Test for Terpenoids
2 mL of plant extract was taken in a test tube. 2 mL of each of chloroform, acetic anhydride and concentrated sulphuric acid was added to it. The formation of blue green rings in the extracts, as a result of Lieberman Burchardt test, showed the presence of terpenoids [27].
Antimicrobial screening
Microbial strains
The tested bacterial strains E.coli, S.aureus, P. vulgaris, S. typhi, Shigella, Citrobacter, Enterobacter, S. pyrogenes, V. cholera and Klebsiella spp., and fungal strains A. niger, Cladosporium, Rhizoctonia, Alternaria, Trichoderma, Penicillium, Curvularia, Paecilomyces and Rhizopus were acquired from Food Microbiology Laboratory (PCSIR), Peshawar. The bacterial and fungal isolates were revived on nutrient agar (NA) and potato dextrose agar (PDA), respectively. Periodic sub-culturing of the cultures was done for their maintenance and these were preserved at 28°C prior to use.
Agar well diffusion method
Antibacterial and antifungal activities of the methanolic extracts of leaves, stem and roots of B.aristata were tested using agar well diffusion method. 10 mg/mL extracts were prepared by dissolving 10 mg of each plant extract in 1 mL of dimethyl sulfoxide (DMSO). The fresh bacterial and fungal strains were then carefully swabbed over the respective media plates using sterilized cotton swabs for a uniform distribution of the microbial cultures. Using sterile cork-borers, 6mm diameters wells were made in these inoculated plates and were filled with 80 µL of each extract via a micropipette. DMSO and Amoxicillin were used as negative and positive control, respectively, for bacterial cultures while for fungal strains, DMSO and Fluconazole were used. The plates for bacteria were incubated overnight at 37°C and those for fungi at 28°C for 72 hours. Following incubation, the antimicrobial activities were evaluated for each extract by measuring, in millimeters, the diameter of the zones of inhibition around each well. Each assay was performed in triplicates and means were calculated [29,30].
Phytotoxic Activity
The phytotoxic activity of the roots, stems and leaves of Berberis aristata was evaluated against Lemna minor [21]. E-media of pH 5.5-6.5 was prepared. 1 mg/mL stock solutions of methanolic plant extracts were prepared in pure DMSO from which solutions of dose concentrations of 10, 100 and 1000 μg/mL were made [31]. To each labelled, sterilized flask (100mL), 20 mL of E-medium was added followed by ten healthy fronds of Lamna minor and finally, the desired dose concentration was added. Paraquat and DMSO were used as negative and positive controls, respectively. After tight plugging with sterilized cotton, the flasks were maintained for seven days at 30°C. The fronds were then subjected to visual examination and the percentage of phytotoxicity was calculated using the following formula:
Percentage of survival = (total no. of leaves – no. of affected leaves × 100
No. of unaffected leaves in control
Percentage of Phytotoxicity = 100 – Percentage of survival
Data Analysis
Statistical analysis of the data was done using Microsoft Excel 2013. The data is expressed as mean ± SEM.
Results
Phytochemical Analysis
The preliminary qualitative screening of the extracts showed positive tests for all phytochemicals except tannins. However, the leaf extract also showed the absence of alkaloids and steroids. (Table 1)
Antibacterial Activity
The tested bacterial strains, assayed alongside with DMSO and amoxicillin, exhibited variable susceptibility to all the extracts of B. aristata, as illustrated in figure 1. The root extract was most active against Shigella and V. cholera (29± 0.57 mm) while the stem and leaf extracts showed maximum inhibitory activity against P. vulgaris (22± 0.00 mm) and S. aureus (29± 0.57 mm), respectively.
Antifungal Activity
The quantitative assessment of the zones of inhibition revealed the antifungal potential of the extracts against all the tested fungal species, with the exception of leaf extract that showed no inhibitory activity against A. niger, Curvularia, Paecilomyces and Rhizopus. The results of antifungal activity are summarized in figure 2.
Maximal inhibition of Curvularia (30± 0.57 mm) and Trichoderma (36± 0.57 mm) was observed with the root and stem extracts, respectively. The leaf extract showed maximum antifungal potential against Trichoderma with a zone of inhibition of 19± 0.57 mm.
Phytotoxic Activity
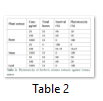
Phytotoxic screening of crude methanolic extracts of B. aristata was done using Lemna minor as test plants. These were monitored regularly and the percentage results calculated after seven days were found to be dose dependent; the activities increasing with the increase in concentration of extracts. All the extracts demonstrated 100% activity at 1000 μg/mL but low activity (10-20%) was observed at 10 μg/mL and 100 μg/mL, respectively. The stem extract, however, did not show any activity at 10 μg/mL. (Table 2)
Tables & Figures
Discussion
In the current study, the plant B. aristata, selected for its medicinal recognition, was subjected to phytochemical and biological (anti-microbial and phytotoxic) investigations. The phytochemical analysis of the methanolic extracts of leaves, stems and roots of B. aristata showed the presence of active compounds such as alkaloids, reducing sugars, steroids, flavonoids, terpenoids, glycosides and saponins while tannins were found to be absent. The results are in accordance with other studies conducted worldwide [11,17,32,33], with the exception of the absence of tannins in the tested methanolic extracts. Also, alkaloids and steroids were not determined in the leaves. Nevertheless, the presence of these secondary metabolites in the different plant extracts have been reported to have multiple biological effects [11] and possibly attribute to the antimicrobial activity shown by the plant, thereby underlying its potential clinical relevance. Further studies can be done to test the antimicrobial activity of the extracts with other extraction solvents and identification of more novel biologically active compounds can be done via FTIR (Fourier Transform Infrared Spectroscopy) analysis.
The results also confirmed the antimicrobial activity of B. aristata which is in well agreement with its wide ethnobotanical uses for treating a large number of ailments [11]. Variable activities of the plant roots, stems and fruits have been reported against a number of pathogenic bacteria and fungi [34,35]. The findings of the present study revealed that the three extracts assayed at a concentration of 10mg/mL showed strong antimicrobial activity against most of the tested bacteria and fungi, that correlates well with previous studies carried out against different bacteria [36-38]. The results of antibacterial potential of the three extracts was found to be in the order of: root extract > stem extract > leaf extract. The root extract showed significant inhibition against all the tested bacterial strains (zones of inhibition of 0-29 mm) as compared to the positive standard (amoxicillin) used (zones of inhibition of 0-22 mm). The stem and leaf extracts showed comparatively small antibacterial potential, with zones of inhibition of 0-22 mm and 0-20 mm, respectively. The results of the study showed much more effectiveness of the B. aristata extracts against bacterial strains as compared to other published works [17,39] which might be ascribed to the differences in procedures used, geographical settings etc.
The methanolic plant extracts showed potential antifungal activity against the tested fungal strains as revealed by the correlation of results with that of positive control, fluconazole. Root and stem extracts were equally active against the employed strains, with their respective zones of inhibition of 0-30 mm; while 0-19 mm zones of inhibition were observed with the leaf extract. However, A. niger, Curvularia, Paecilomyces and Rhizopus were not inhibited by the leaf extract at the assayed concentration. Thus, the promising antimicrobial activity of the extracts furnishes strong support for the various traditional uses of this plant in medicinal applications.
The grave concern of reduction in the yield and quality of agricultural crops due to weeds has laid much emphasis on the natural plant based allelochemicals for potential weed control [40]. In this study, the B. aristata extracts, analyzed for phytotoxic activity against Lemna minor, inhibited the growth of test plants, thereby demonstrating the plant’s allelopathic potential. Our findings are in strong accordance with the results of previous studies carried out using Arceuthobium oxycedri, Phyllanthus muellerianus and Zizyphus jujube [23,24,41]. The inhibition of weed was concentration dependent as percentage phytotoxicity increased with an increase in concentration. 100% inhibition was recorded at 1000 µg/mL which affirms the potentially high phytotoxicity of B. aristata and proposes its use as a natural herbicide for weed control in crops of economic importance.
The present study revealed remarkable antibacterial and antifungal activities of the methanolic B.aristata extracts against the tested microbial isolates, with results higher than and/comparable to that of the standard drugs (amoxicillin and fluconazole) used. The preliminary phytochemical screening of the extracts showed the presence of secondary metabolites to which the antimicrobial activity demonstrated by the plant might be attributed to. Thus, this plant can be used as a potential alternative treatment against drug resistant pathogens. The use of purified extracts, however, will help in improving their sensitivity towards selected microbes. Also, the plant showed excellent phytotoxicity against Lemna minor, thereby suggesting its role in the significant management of weeds in an environmentally safe manner. However, further studies are required to decipher its phytotoxic mechanism and to explicitly investigate its efficiency as a potential herbicide and a disease control agent.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Azwanida N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants, (2015); 4(196): 2167-0412.1000196.
- Popović Z, Matić R, Bojović S, Stefanović M, Vidaković V. Ethnobotany and herbal medicine in modern complementary and alternative medicine: An overview of publications in the field of I&C medicine 2001–2013. Journal of ethnopharmacology, (2016); 181182-192.
- Bilal M, Rasheed T, Iqbal HM, Hu H, Wang W, et al. Macromolecular agents with antimicrobial potentialities: A drive to combat antimicrobial resistance. International Journal of Biological Macromolecules, (2017); 103: 554-574.
- Gul F, Shinwari ZK, Afzal I. Screening of indigenous knowledge of herbal remedies for skin diseases among local communities of North West Punjab, Pakistan. Pakistan Journal of Botany, (2012); 51609-1616.
- Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, Linder T, Wawrosch C, et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnology advances, (2015); 33(8): 1582-1614.
- Mothana RA, Lindequist U. Antimicrobial activity of some medicinal plants of the island Soqotra. Journal of ethnopharmacology, (2005); 96(1): 177-181.
- Ali M, Malik A, Sharma KR. Vegetative propagation of Berberis aristata DC. An endangered Himalayan shrub. Journal of Medicinal Plants Research, (2008); 2(12): 374-377.
- Srivastava S, Rawat A. Quality evaluation of ayurvedic crude drug daruharidra, its allied species, and commercial samples from herbal drug markets of India. Evidence-Based Complementary and Alternative Medicine, (2013); 2013.
- Pareek A, Suthar M. Antidiabetic activity of extract of Berberis aristata root in streptozotocin induced diabetic rats. Pharmacologyonline, (2010); 2179-185.
- Anubhuti P, Rahul S, Kant KC. Comparative study on the antimicrobial activity of Berberis aristata from different regions and berberine in vitro. International journal of life science and Pharma Research, (2011); 1(1): 17-20.
- Shahid M, Rahim T, Shahzad A, Latif T, Fatma T, et al. Ethnobotanical studies on Berberis aristata DC. root extracts. African Journal of Biotechnology, (2009); 8(4): 556-563.
- Potdar D, Hirwani R, Dhulap S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia, (2012); 83(5): 817-830.
- Affuso F, Mercurio V, Fazio V, Fazio S. Cardiovascular and metabolic effects of Berberine. World journal of cardiology, (2010); 2(4): 71.
- Kumar A, Chopra K, Mukherjee M, Pottabathini R, Dhull DK. Current knowledge and pharmacological profile of berberine: an update. European journal of pharmacology, (2015); 761288-297.
- Ahmed T, Abdollahi M, Daglia M, Nabavi SF, Nabavi SM. Berberine and neurodegeneration: A review of literature. Pharmacological Reports, (2015); 67(5): 970-979.
- Das S, Das MK, Mazumder PM, Das S, Basu S. Cytotoxic activity of methanolic extract of Berberis aristata DC on colon cancer. Global Journal of Pharmacology, (2009); 3(3): 137-140.
- Lamichhane B, Adhikari S, Shrestha P, Shrestha BG. Study of phytochemical, antioxidant, antimicrobial and anticancer activity of Berberis Aristata. Journal of Tropical Life Science, (2014); 4(1): 01-07.
- Tiwari BK, Khosa R. Evaluation of the hepatoprotective and antioxidant effect of Berberis asiatica against experimentally induced liver injury in rats. International Journal of Pharmacy and Pharmaceutical Sciences, (2010); 2(1): 92-99.
- Rizwana K, Gilani S. Conservational status of plant seedlings in Ayubia National Park, Pakistan. Lyonia, (2005); 8(1): 51-60.
- Ibrar M, Muhammad N. Evaluation of Zanthoxylum armatum DC for in-vitro and in-vivo pharmacological screening. African Journal of Pharmacy and Pharmacology, (2011); 5(14): 1718-1723.
- Choudhary MI, Thomsen WJ Bioassay techniques for drug development. (2001); Taylor & Francis.
- Khan RA, Khan MR, Sahreen S, Bokhari J. Antimicrobial and phytotoxic screening of various fractions of Sonchus asper. African Journal of Biotechnology, (2010); 9(25): 3883-3887.
- Ahmad B, Khan I, Bashir S, Azam S, Hussain F. Screening of Zizyphus jujuba for antibacterial, phytotoxic and haemagglutination activities. African Journal of Biotechnology, (2011); 10(13): 2514-2519.
- Zaidi MA, Huda A, Crow Jr SA. Pharmacological Screening of Arceuthobium oxycedri(Dwarf Mistletoe) of Juniper Forest of Pakistan. Online Journal of Biological Sciences, (2006); 6(2).
- Hoque N, Imam MZ, Akter S, Mazumder M, Hoque E, et al. Antioxidant and antihyperglycemic activities of methanolic extract of Glinus oppositifolius leaves. Journal of Applied Pharmacutical Sciences, (2011); 1(7): 50-53
- Tofighi Z, Ostad S, Khezrrahdoost S, Salehizadeh H, Yassa N. Potent anti-nociceptive and anti-inflammatory effects of methanol fraction of Otostegia persica extract and its components. Research Journal of Pharmacognosy (RJP), (2017); 4(2): 23-29.
- Gracelin DHS, Britto A, Kumar B. Qualitative and quantitative analysis of phytochemicals in five Pteris species. International Journal of Pharmaceutical Sciences and Research, (2013); 5105-107.
- Sofowora A. Recent trends in research into African medicinal plants. Journal of ethnopharmacology, (1993); 38(2-3): 197-208.
- Singh M, Srivastava S, Rawat A. Antimicrobial studies of stem of different Berberis species. Natural Product Science, (2009); 15(2): 60-65.
- De Britto AJ, Jeya P, Kumar R, Gracelin S, Herin D. Abrus precatorius L.: A medicinal plant with potential as antibacterial agent. Journal of Pharmacy Research, (2012); 5(2): 1207-1209.
- Khuda F, Iqbal Z, Khan A, Nasir F, Muhammad N, et al. Metal analysis, phytotoxic, insecticidal and cytotoxic activities of selected medicinal plants of Khyber Pakhtunkhwa. Pakistan journal of pharmaceutical sciences, (2012); 25(1).
- Mittal M, Juyal V, Singh A. Phytochemical, antidiabetic, and cytoprotective properties of Berberis aristata DC. Root Extracts. Pharmaceutical Crops, (2012); 364-68.
- Saied S, Batool S, Naz S. Phytochemical studies of Berberis aristata. Journal of Basic and Applied Sciences, (2007); 1-3.
- Singh M, Srivastava S, Rawat A. Antimicrobial activities of Indian Berberis species. Fitoterapia, (2007); 78(7): 574-576.
- Malik F, Mirza T, Riaz H, Hameed A, Hussain S. Biological screening of seventeen medicinal plants used in the traditional systems of medicine in Pakistan for antimicrobial activities. African Journal of Pharmacy and Pharmacology, (2010); 4(6): 335-340.
- Komal S, Ranjan B, Neelam C, Birendra S, Kumar SN. Berberis aristata: A review. International Journal of Research in Ayurveda and Pharmacy, (2011); 2(2): 383-388.
- Mazumder PM, Das S, Das MK. Phyto-pharmacology of Berberis aristata DC: a review. Journal of Drug Delivery and Therapeutics, (2011); 1(2): 46-50.
- Sharma C, Aneja K, Kasera R. Screening of Berberis aristata DC. for antimicrobial potential against the pathogens causing ear infection. International Journal of Pharmacology, (2011); 7(4): 536-541.
- Wagh S, Vidhale N. Antimicrobial activity of Berberis aristata against some human pathogenic bacteria and fungi. Bioscience, Biotechnology, and Biochemistry, (2010); 338-42.
- Batish DR, Kaur M, Singh HP, Kohli RK. Phytotoxicity of a medicinal plant, Anisomeles indica, against Phalaris minor and its potential use as natural herbicide in wheat fields. Crop Protection, (2007); 26(7): 948-952.
- Onocha P, Ali M. Antileishmaniasis, phytotoxicity and cytotoxicity of Nigerian Euphorbiaceous Plants 2: Phyllanthus amarus and Phyllanthus muellerianus Extracts. African Scientist, (2010); 11(2): 79-83.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0/