Full Length Research Article
Biological Activity and Characterization of Bioactive Compounds under Lead Induced Stress in Maize
Javed Iqbal Wattoo1*, Saba Munawar1, Muhammad Afzal1, Amjad Farooq2, Mushatq A. Saleem1
Adv. life sci., vol. 5, no. 3, pp. 96-103, May 2018
*- Corresponding Author: Javed Iqbal Wattoo (Email: javed.cgiar@gmail.com)
Authors' Affiliations
2- Department of Environmental Sciences, COMSATS Institute of Information Sciences (CIIT), Vehari, 61100, Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Lead is most commonly released environmental contaminant making its way to air, soils and water. It causes hormonal imbalance and over production of reactive oxygen in plants when absorbed through leaves and roots. It contaminates the ground water depending on the type of soils and characteristics of lead. Plants ability to tolerate lead is linked with cell wall potential, activation of antioxidants defense mechanism and synthesis of osmolytes.
Methods: The study was designed to evaluate the effects of Pb(NO3)2 induced stress on biological activity and bioactive compounds in maize. The plants were subjected under two different lead concentrations (T1- 0.35mg/ml and T2- 0.45mg/ml). Phytochemical screening revealed the presence of alkaloids, coumarins, saponins, tannins and terpenoids in maize. Total Phenolic Content (TPC) was increased (T1- 45%, T2- 58.42%) under lead stress when compared with control (36.29%). The cytotoxicity was checked using hemolytic activity against human red blood cells.
Results: The scavenging rate was highest (T1- 33.5%, T2- 52%) when compared with control (18.6%). Zone of inhibition of Aspergillus niger was highest amongst other fungal strains. The HPLC results showed that maize has some phyto-ingredients which may be accountable for cell reinforcement and anti-microbial activity. The extracts were further analyzed for the biochemical profile like superoxide dismutase, peroxidase, catalase, amylase and protease. Escherichia coli showed maximum activity with control (25±3.46mm) and maximum under stress (T1- 17±1.633 mm, T2- 20±4.08 mm).
Conclusion: Lead stress altered all the activities when compared to control plants. In conclusion, Maize can be used as a potential indicator for lead and other compounds to play a vital role in phytoremediation. The results would further lead to find the new compounds and plant mechanism to cope with stress.
Keywords: Maize, Lead stress, HPLC, Biological activity, bioactive compounds, Antimicrobial activity
Maize (Zea mays L.) plays a key role in human nutrition and farm feeds. It is also an important component of cropping systems of many countries including Pakistan with significant share in the industry [1]. It is prominent as a diet of many people in different forms like corn-flour, popcorn, whole corn, corn-oil, starch, syrup, cornmeal and flakes [2].
Environmental contamination is a global threat because of untreated industrial wastes and use of hazardous chemicals (Pesticides & Insecticides) in Agro-farming. These activities discharge toxic chemicals directly to the environment. Heavy metals are elements with higher density and are highly toxic for human health at higher concentration. These heavy metals are not biologically degraded in soil [3] causing disastrous effects on plant productivity, human and animal health. Lead is a lethal pollutant to be absorbed by plants from contaminated soils and heavy metal impurities [4]. Plants absorb elements from soil; some are referred as essentials because they are important for plants to complete their life cycle, some are known to be toxic at low concentrations; these are heavy metals i.e. chromium, mercury, cadmium, arsenic and lead [5]. It drastically reduces different growth phases in plants such as germination, mitosis, respiration, leaf chlorosis, root-shoot development, photosynthesis and enzymatic activities [6]. Lead contamination has picked up an extensive consideration as a strong environmental pollutant. Environment Protection Agency (EPA) reported lead as most common heavy metals in the environment [7].
Among different heavy metals, lead is the second most harmful pollutant after arsenic [8]. It often leads to hormonal imbalance and induce over production of reactive oxygen species in plants. Lead being non-redox metal, cause ROS production that leads to oxidative stress in plants. Once produced, ROS readily attacks to biological structures and biomolecules and results in metabolic dysfunction [9]. Some plants species tolerate lead through inactivation & complexation i.e. Allium cepa, Hordeum vulgare and Zea mays, other species experience toxicity like Brassica napus and Phaselous valgaris because lead hampers some metabolic pathways [10]. Plants have three mechanisms of lead tolerance i.e. (i) Activation of physical barriers against lead uptake by passive mechanism. (ii) Metal detoxification and its excretion to extra cellular spaces, by inducible mechanism and (iii) Activation of anti-oxidative defense system [11]. Anti-oxidants i.e. SOD, POD and CAT are involved in detoxification of ROS in plants [12]. SOD activity have been observed in a variety of plants exposed to a broad range of stresses, such as drought, temperature, pathogen infection and heavy metal exposure [13].
The present study was designed under the following objectives (a) To characterize bioactive compounds of maize under lead induced stress using HPLC (b) To study biological activity of maize using different microbial strains (c) To find the variant levels of SOD, POD, amylases, catalases, total phenolic contents and antioxidant assays of maize extracts under lead stress.
Plant materials and extract preparation
The study was conducted at Department of Biotechnology, University of Central Punjab, Lahore. Maize seeds were collected from Maize and Millet Research Institute (MMRI), Sahiwal. Experiment was conducted in plastic pots with two treatments. Lead Nitrate was used as the source of lead. Separate treatments were conducted with the half strength of Hoagland’s solution within interval of three days, till the end of the trials. Following treatments were applied; Treatment 1 had 350ppm lead; Treatment 2 with 450ppm lead and control plants without lead. After 30 days, plants were harvested. About 0.5 grams of each plant sample was grinded in pestle and mortar with Liquid Nitrogen. The 2mL Phosphate buffer was added in the extract time to time. The extract was collected in the eppendorf and centrifuged at 14000 rpm for 10 min at 4°C. Supernatant was collected, labelled and preserved in the freezer.
Identification of Phytochemicals
Alkaloids test
To identify the presence of alkaloids, 4mL of 1% HCl was added to the 0.25 g of plant extract followed by heating and filtration. In 1mL of filtrate, 6 drops of Mayor’s reagents was added separately. Creamish precipitate/orange precipitate indicated the presence of respective alkaloids. Coumarins test was performed by dissolving 1mL of plant extract with 10% NaOH. Ultraviolet light for yellow florescence was inspected and recorded. Quinones test was conducted by adding few drops of conc. H2SO4 in 1mL of extract and absorbance of red color was recorded for the presence of quinones. Similarly, to find the saponins, Saponins test was performed by mixing 1mL of maize extract in 5 mL of distilled water followed by shaking to create stable persistent foam. The presence of tannins was checked by Tannins test by adding 1mL of plant extract in 5mL of distilled water and further adding 3 drops of 10% lead acetate till the appearance of white precipitation. Terpenoids test was performed by mixing 2mL of plant extract in 2mL of chloroform followed by filtration. Same volume of acetic acid and a drop of concentrated H2SO4 was mixed till the appearance of green rings.
Determination of Total Phenolic Contents
Total Phenolic Contents (TPCs) were determined following the Follin-Ciocalteu (F–C) assay as described [14]. 1 mg/mL of extract was dissolved in 0.5mL Follin reagent and mixed in 7.5mL of distilled. The mixture was kept at normal temperature for 10 minutes. 1.5mL of 20% sodium carbonate was added in the solution and incubated for twenty minutes at 40°C. Absorbance was recorded at 755nm using spectrophotometer.
Hemolytic Activity
Cytotoxicity was studied by hemolytic activity of extract [15]. 1mg/mL extract was added in 10% DMSO. 3mL of fresh human blood was taken in heparinized tubes, tenderly blended and filled into 15mL falcon tube. The samples were centrifuged at 10000rpm for five minutes and supernatant was poured off. RBCs were washed thrice with chilled isotonic phosphate saline buffer solution (pH 7.4). Diluted blood cell suspension was mixed with maize extract and incubated at 37°C for 35 minutes. After incubation and agitation, tubes were put on ice for five minutes and again centrifuged for seven minutes at 13000rpm. Supernatant was taken and diluted with chilled PBS. O.1% Triton X-100 was taken as positive control while PBS as negative. Absorbance was measured at 576nm.
Enzymatic Activity
Superoxide dismutase
The Superoxide dismutase was measured following Beyer and Fridovich method using spectrophotometer at 560 nm. The reaction mixture contained 50 mM of potassium phosphate buffer (pH 7.2), 50 μM NBT and 0.01M EDTA. 0.745g of methionine was mixed in 100mL distilled water followed by addition of riboflavin (0.2 mM). Different concentrations of maize extract (50, 100, 150, 200)µL were added in test tubes with equal diameter of 100µL of the extraction buffer, 100µL riboflavin, 100µL EDTA and 100µL L-Methionine in all tubes. The reaction was started by adding riboflavin and placing the tubes under 15W fluorescent lamps for 15 min. A complete reaction mixture without extract was used as control.
Peroxidase Activity
The POD activity was measured using pyrogallol as the substrate. Reaction mixture comprising 100Mm potassium phosphate buffer, with pH 6.0, 0.50% (w/w) Hydrogen Peroxide Solution 1:6 dilution using hydrogen peroxide solution 30% (w/w) in ultrapure water, 5% (w/v) pyrogallol solution 50 mg/mL solution using pyrogallol in ultrapure water. Different concentrations of plant extracts (50, 100, 150, 200µL) were added in the test tubes with same diameter of 300µL of the extraction buffer, 300µL pyrogallol solution, 100µL peroxide solution and 100µL distilled water. Absorbance was measured at 420nm. Similarly, Catalase activity analysis was conducted by the technique of Aebi (1994). 50mM phosphate buffer (pH 7.0) and 30% hydrogen peroxide were freshly made. Extract solution (50, 100, 150 and 200µL) was placed in tubes and mixed with 1mL phosphate buffer and 1mL of hydrogen peroxide. The decomposition of hydrogen peroxide was measured at 240 nm. 1 unit of catalase was taken as the amount of enzyme required to decrease the absorbance at 240 nm by 0.05 unit/minute. Amylase activity was measured by Rick and Stegbauer method devised in 1974. The method was performed by mixing 1mL of 1% starch in 1mL of sodium phosphate buffer and 1mL of sodium potassium tartrate solution followed by incubation at 25°C for three minutes. 2mL of dinitrosalicylic acid was mixed and allowed to boil for 5 minutes. It was diluted with 16mL distilled water after cooling. The absorbance was measured at 540nm. One unit of amylase was taken as the amount of enzyme causing the release of 1µmole of reducing sugar in 1 minute. Similarly, Protease activity was measured as described [16]. The reaction mixture contained 2mL of 1% casein in NaOH buffer 10% TCA, 0.1M of glycine NaOH buffer and 1mL of enzyme solution incubated at room temperature for 20 minutes. 3mL of TCA was added in former mixture which stopped the reaction. Absorbance was measured at 280nm.
Antioxidant assay using DPPH method
DPPH radical scavenging activity was evaluated by 0.001g of DPPH in 50mL methanol. Different concentrations of plant extracts (50, 100, 150, 200µL) were added in the test tubes with same diameter of 700µL of the DPPH solution. Mixture was shaken overwhelmingly and left for 20 minutes in dim light. The absorbance was measured at 517nm against a solvent blank. The scavenging % of DPPH radicals was computed according to the formula as follows:
Scavenging rate (%) = [(Ao -A1)/Ao] x 100
Where, Ao means absorbance of control
A1 means absorbance of extracts in DPPH solution
Antimicrobial Activity
Plant extracts were tested against different microorganisms. The extracts exhibited antimicrobial activity against most of bacterial and fungus strains tested. Bacterial strains used were Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Streptococcus faecalis while three fungus cultures Aspergillus falvus, A.niger and A. fumigatus. Nutrient agar was used as the culture media. From the cultures of P. aeruginosa, S. aureus, E. coli, S. faecalis, A. falvus, A.niger and A. fumigatus, slants were made and incubated at 37°C for one day. On other day, agar and culture suspension was added in sterilized petri plates and mixed well for equal distribution of microbial cells in medium. Seven plates with agar medium was filled and culture suspension to solidify. After the solidification of medium, discs were put in the plates. 100µL of extract induced with lead stress along with positive and negative controls. Bacterial plates were incubated at 37°C for one day while fungus plates were placed at 27°C for 3-4 days. After 24 hours, plates were observed for zone of inhibition. The diameter of zone of inhibition was measured with the help of vernier caliper in mm. The actual measurement of zone of inhibition was obtained after subtracting the value produced by pure solvent.
High-Performance Liquid Chromatography analysis (HPLC)
HPLC analysis of the samples was performed on Shim-pack CLC-ODS-C18 column (25 cm × 4.6 mm, 5 µm), using a gradient program with two solvent systems, A was taken as equal to (H2O: AA-94: 6, pH- 2.27) and B (CAN 99.99 %), 0.1-15 minutes = 14.9% B, 16-29 = 46 % B at a constant solvent flow speed 1mL/minute. The signal was detected at 280 nm by UV- Visible detector.
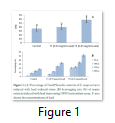
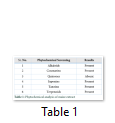
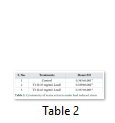
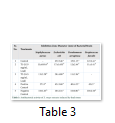
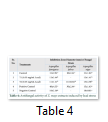
Extracts of maize plants under lead stress revealed the presence of secondary metabolites like alkaloids, coumarins, saponins, tannins and terpenoids. Yellow inflorescence in UV light indicated that coumarins were present in plant sample. The presence of blue green ring indicated positive results for terpenoids. White precipitations indicated that tannins were also present in the sample while quinones were absent (Table 1). Yellow inflorescence in UV light indicated the presence of coumarins while presence of blue green ring indicated positive results for terpenoids. White precipitation indicated that tannins were also present in the sample. The Total Phenolic Content of maize extract induced with lead stress showed that it has more TPC% as compared to control (Fig. 1A). The TPC% of control, Treatment 1 and Treatment 2 were 36.29, 40.22 and 58.42, respectively. It showed lead stress enhanced the TPC of maize (Fig. 1B). Maize plant is known as a rich source of phenolic and antioxidants [17]. The cytotoxicity of maize extract was assayed using in vitro hemolytic activity against human blood erythrocytes and plant showed minor cytotoxicity when compared with control (Table 2). Dealing of cells with cytotoxic combinations causes diverse harm in humans. Cell may experience loss of cell membrane integrity and expire promptly because of cell lysis [18]. While evaluating for cytotoxicity, automated permanence of cell membrane is a good sign of assessment of effects of various compounds for cytotoxicity [19]. The samples showed scavenging values ranging from 1.8–52%. The scavenging rate (%) of control was 1.8 –18.6%, Treatment 1 was 5.8–33.5% and Treatment 2 was 21.6–52%. The highest percentage was observed in Treatment 2. The Scavenging rate was observed to be increased with increase in lead quantity. The results of biological activity showed that the Z. mays extracts contain antimicrobial properties against selected bacterial and fungal strains (Table 3).
Staphylococcus aureus was found resistant to control while Streptococcus faecalis was resistant to Treatment 2. Escherichia coli had the maximum activity towards control while it showed less activity in case of lead stress treatments. Likewise, the antifungal activity exhibited different sensitivities. Root extract indicated action against all the pathogen bacteria including B. subtilis, S. typhi and S. aureus but it showed less inhibition against E. coli and M. leteus. Antioxidant activity of the non-refined extracts figured as far as TAC, TPC and TFC. ‘Agar diffusion method’ was used to check antimicrobial activity. All extracts showed activity against E. coli and S. aureus. The size of the inhibition zones ranged between (11.20±0.23) mm to (26.10±2.09) mm (P<0.05) with turmeric and cinnamon being the best against S. aureus while garlic as least effective for E. coli.
While three strains of fungus Aspergillus falvus, Aspergillus niger and Aspergillus fumigatus were tested, Aspergillus fumigatus showed good results towards Treatment 1. Zone of Inhibition of Aspergillus niger was highest amongst other two strains. These extracts exhibit good antimicrobial activities. The results showed that different species exhibited different sensitivities towards the Z. mays extract induced with lead stress. Aspergillus falvus was found resistant against treatment 1, while it had more Zone of Inhibition in contrast to control.
Tables & Figures
Stress is the major cause of oxidative damage to plants [20]. However, plants can develop a strong defense system against the damaging effects. The defense system includes SOD, POD, CAT [21]. The results of current study revealed that lead stress increased the activity of SOD, POD, CAT, Amylase as well as Protease when compared with control. The concentration of 200µL in both treatments enhanced the activity of SOD in contrast to 50µL under lead stress.
The antioxidant activity of enzymes i.e. SOD, POD, CAT, Amylase and Protease increased significantly in both Treatments (Fig.2). SOD catalyzed the dismutation of superoxide into Hydrogen peroxide and Oxygen and is the most effective antioxidant enzymes to save it from damaging. SOD is the first line of defense against ROS caused by environmental stresses being associated with increased stress tolerance [22]. Likewise the activity of POD increased significantly with concentration of 200µL in both treatments under lead stress as compared to control. We observed significant variations among defense system and found that enzymatic and non-enzymatic anti-oxidants activities were dependent on Lead-concentration.
In current study, eight phenolic compounds were detected. Five compounds in T1, quercetin, caffeic acid, chlorogenic acid, p-coumaric acid and m-coumaric acid. Among these five compounds, caffeic acid was present in larger concentration (15.71 ppm) followed by chlorogenic acid (8.20 ppm), m-coumaric acid (3.60 ppm), p-coumaric acid (1.53 ppm) and quercetin (0.91 ppm). In T2, quercetin, caffeic acid, chlorogenic acid, syringic acid and ferulic acid were present. Among these five compounds, ferulic acid was present in larger concentration (27.43 ppm) followed by chlorogenic acid (8.76 ppm), caffeic acid (4.40 ppm), syringic acid (1.94 ppm) and quercetin (1.26 ppm). While three phenolic compounds were present in control i.e. quercetin, gallic acid and syringic acid. Among these three compounds, syringic acid was present in larger concentration (4.49 ppm) followed by gallic acid (1.96 ppm) and quercetin (1.00 ppm).
Crop productivity is affected by many biotic and abiotic stresses limiting plant growth and development. The current experiment proved the presence of phytochemicals, total phenolic contents, minor cytotoxicity, antimicrobial activity and enzymatic antioxidants like SOD, POD, CAT, amylase and protease in maize under lead stress. All the parameters somehow changed due to lead stress which enhanced the levels of antioxidants in treated plants when compared with control. Plants have internal system of regulation to modify these responses. A complex mixture of phytochemicals present in plants and their products is responsible for the potent antioxidant activities. Maize can be used as an indicator species for lead. Antioxidants present in maize might play a key role in the detoxification of lead. Furthermore, study of genetic basis of these compounds using cutting edge sequencing and mapping techniques would lead to dissect the mechanism of inheritance of genes controlling lead stress.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Rose DJ, Inglett GE, Liu SX. Utilization of corn (Zea mays L.) bran and corn fiber in the production of food components. Journal of Science of Food and Agriculture, (2010); 90(6): 915-924.
- Ranum P, Peña‐Rosas JP, Garcia‐Casal MN. Global maize production, utilization, and consumption. Annals of the New York Academy of Sciences, (2014); 1312: 105-12.
- Wolz S, Fenske RA, Simcox NJ, Palcisko G, Kissel JC. Residential arsenic and lead levels in an agricultural community with a history of lead arsenate use. Journal of Environmental Research, (2003); 93(3): 293-300.
- Domy C, Adriano. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals. 2nd Edition, Springer, New York, 867.
- Peralta JR, Lopez ML, Narayan M, Saupe G, Gardea J. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. International Journal of Biochemistry and Cell Biology, (2009); 41(8-9): 1665–1677.
- Ekmekci Y, Tanyolac D, Ayhan B. A crop tolerating oxidative stress induced by excess lead: maize. Acta Physiologiae Plantarum, (2009); 31(2): 319-30.
- Islam E, Yang X, Li T, Liu D, Jin X, Meng F. Effect of lead toxicity on root morphology, physiology and ultra-structure in two ectypes of Elsholtziacirgyi. Journal of Hazardous Materials, (2007); 147(3): 806-816.
- Srivastava D, Ajeet S, Mamta B. Lead toxicity and tolerance in plants. Journal of Plant Science and Research, (2015); 2(2):123.
- Shahid M, Pinelli E, Pourrut B, Silvestre J, Dumat C. Lead-induced genotoxicity to Viciafaba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicology and Environment Safety, (2011); 74: 78–84.
- Wierzbicka M. Comparison of lead tolerance in Allium cepa with other plant species. Journal of Environmental Pollution, (1999); 104: 41-52.
- Ashraf AS, Mo ZW, Hussain S, Anjum SA, Khan I. Lead toxicity in rice; effects, mechanisms and mitigation strategies-a mini review. Journal of Environmental Science, (2015); 22(23): 18318–18332.
- Mittler R. Oxidative stress, antioxidant and stress tolerance. Journal of Trends Plant Science, (2002); 7(9): 841–851.
- Pawlak S, Firych A, Rymer K, Deckert J. Cu, Zn-superoxide dismutase is differently regulated by cadmium and lead in roots of soybean seedlings. Acta Physiologiae Plantarum, (2009); 31(4): 741–747.
- Hu QP, Xu JG. Profiles of carotenoids, anthocyanins, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. Journal of Agriculture Food Chemistry, (2011); 59(5): 2026–2033.
- Powell WA, Catranis CM, Maynard CA. Design of self-processing antimicrobial peptides for plant protection. Letters of Applied Microbiology, (2000); 31(2): 163-168.
- Yamada T, Atsushi K, Hiroyuki O, Tatsuru M, Hiroshi S, Kenichiro T. Isolation of the Protease Component of Maize Cysteine Protease-Cystatin Complex: Release of Cystatin is not crucial for the activation of cysteine protease. Journal of Plant Cell Physiology, (2001); 42(7): 710-716.
- Dong J, Cai L, Zhu X, Huang X, Yin T, Fang H, Ding Z. Antioxidant activities and phenolic compounds of cornhusk, corncob and Stigma maydis. Journal of Brazilian Chemistry, (2014); 25(11): 1956–1964.
- Baillie JK, Thompson AA, Irving JB, Bates MGD, Sutherland AI, Macnee W, Maxwell SRJ, Webb DJ. Oral antioxidant supplementation does not prevent acute mountain sickness: double blind, randomized placebo-controlled trial. International Journal of Medicine, (2009); 102(5): 341–348.
- Sharma P, Sharma JD. In vitro hemolysis of human erythrocytes by plant extracts with antiplasmodial activity. Journal of Ethnopharmacol, (2001); 74(3): 239–243.
- Jalali-e-Emam SMS, Alizadeh B, Zaefizadeh M, Zakarya RA, Khayatnezhad M. Superoxide dismutase (SOD) activity in NaCl stress in salt-sensitive and salt-tolerance genotypes of colza (Brassica napus L.) Middle-East Journal of Science Research, (2001); 7(1): 7-11.
- Joseph B, Jini D. Development of salt stress-tolerant plants by gene manipulation of antioxidant enzymes. Asian Journal of Agriculture Research, (2011); 5(1): 17-27.
- Zhang X, Ervin E, Evanylo G, Sherony C, Peot C. Biosolids impact on tall fescue drought resistance. Journal of Residuals Science and Technology, (2005); 2(3): 173–180.
- Moran JF, Becana MI, Frenchilla S, Klucas RV, Aparicio T. Drought induces oxidative stress in pea plants. Journal of Planta, (1994); 194(3): 346-352.
- Luna M, Badiani M, Felici M, Sermanni GG. Selective enzyme inactivation over water stress in maize (Zea mays L.) and wheat (Triticum aestivum L.) seedlings. Journal of Environmental and Experimental Botany, (1985); 25(2): 153-156.
- Wang Z, Huang B. Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Journal of Crop Science, (2004); 44(5): 729-1736.
- Zhang J, Kirkham MB. Antioxidant responses to drought in sunflower and sorghum seedlings. Journal of New Phytologist, (1996); 132(3): 361-373.
- Feierabend J, Smirnoff N. Catalases in plants: molecular and functional properties and role in stress defense. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell, (2005); 101-140.
- Singh R, Tripathi RD, Dwivedi S, Kumar A, Trivedi PK, Chakrabarty D. Lead bioaccumulation potential of an aquatic macrophyte Najasindica are related to antioxidant system. Journal of Bioresource Technology, (2010); 101(9): 3025–3032.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0