Short Communication
Evaluation of antimicrobial activity of a lichen used as a spice (Platismatia glauca)
Emad M. Abdallah*
Adv. life sci., vol. 6, no. 3, pp. 110-115, May 2019
*- Corresponding Author: Emad M. Abdallah (Email: emad100sdl@yahoo.com)
Authors' Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Lichen is a complex symbiotic relationship arose from algae or cyanobacteria that live together with some fungal species. Some of them are edible and consumed as spice such as Platismatia glauca. The current study aimed to evaluate it’s the antimicrobial properties of the methanolic extract of lichen thalli of P. glauca against some referenced bacterial and yeast strains.
Methods: Disc diffusion test, minimum inhibitory (MIC) and minimum bactericidal (MBC) or minimum fungicidal (MFC) tests were carried out to evaluate the antimicrobial activity of lichen.
Results: All tested microorganisms exhibited varying degrees of susceptibility. Among the tested strains, the most susceptible bacterium -using the disc diffusion assay- was Staphylococcus saprophyticus (18.5±1.0 mm), followed by Staphylococcus aureus (14.5±0.5 mm), Shigella flexneri (12.5±1.5 mm), Streptococcus pneumoniae (12.0±1.0 mm), Proteus vulgaris (11.5±0.5 mm), Salmonella Typhimurium (11.5±0.5 mm), Bacillus cereus (11.0±1.0 mm) and Escherichia coli (11.0±0.0 mm), respectively. It also showed high antifungal activity against Candida albicans (22.5±0.5 mm). The MIC, MBC and MFC were promising, which were as low as 3.125 to 12.5 mg/ml for MIC and 6.25 to 12.5 mg/ml for MBC and MFC.
Conclusion: From the obtained results, it is concluded that the lichen thalli of Platismatia glauca possesses a remarkable antimicrobial activity and it may be considered as a source of potential antimicrobial agents.
Keywords: Antibacterial, Antifungal, Lichen, Platismatia glauca
The eternal conflict between human and pathogens is a never-ending battle. Although, a sweeping victory was achieved after the discovery of antibiotics. The situation soon worsened due to the emersion of mutant pathogens resistant to these antibiotics, which led to calling for adoption of new strategies to invent novel effective antibiotics from natural products [1]. The antimicrobial properties that characterized some natural products are attributed to some bioactive secondary compounds include flavonoids, phenolics, phenolic acids, terpenoids, alkaloids, saponins, tannins, quinones and coumarins [2]. Spices have been used since ancient times as food additives, seasoning, and flavoring agents. A plentiful number of spices showed potent antimicrobial activity such as garlic, cinnamon, clove, thyme, basil, oregano, ginger, pepper, cumin and many more [3]. However, little is known about lichen spices. The symbiotic relationship between an alga and a fungus has led to a new life form known as lichen, which is now classified within the fungus kingdom. This amazing creature is able to grow in a harsh environmental condition and play an important role in the early developmental stages of the environment form primary succession passing to secondary ecosystem succession [4]. Lichen was well known and exploit as dyes, perfumes and remedies since pharaonic civilization. Many lichen species are used in traditional medicine worldwide, and it is thought to treat some diseases and disorders such as pulmonary tuberculosis, bronchitis, dyspepsia, wound healing, bleeding piles, spermatorrhoea, diabetes, sore throat and toothache [5] Platismatia glauca (L.) is a lichen species belong to family Parmeliaceae, one of the most widely distributed families of lichen with about 80 genera and 2700 species, which represents approximately 15% of the known lichens in nature. Members of this family are characterized by foliose shape, cupulate exciple epihymenium, dorsi-ventral fruticose to sub-fruticose thallus and the color of the upper surface are varying from gray, yellow-green, brown to olive-brown [6]. Platismatia glauca are widely traded as a spice, this spice is called "Kalpaasi" in Tamilian cuisine, it is also known in the Middle East as “Shibah”. The aim of this study was to evaluation of the antimicrobial capacity of a popular spice lichen (Platismatia glauca), which are traded worldwide and have entered in food recipes beside some applications in the traditional folk medicine.
Lichen material
Thalli of lichen (Platismatia glauca) (Figure 1) was purchased from a reputed herbal market at Al-Rass town, Saudi Arabia, the seller declared that it is exported from India. It is mainly sold as a spice. Lichen was authenticated at our lab using standard keys [7] and “Lucid keys” available at The British Lichen Society.
Preparation of lichen extract
Dried lichen was mechanically ground and filtered by the refinery to get a fine powder. Twenty grams of this powder was macerated in 200 ml of 80% methanol and mixed well. The mixture was poured in a dark well tighten bottle and kept inside the incubator at 40oC for 3 days, with frequent shaking. Then, the infusion was filtered with multi-layers of muslin cloth first and followed by filtration with Whatman filter paper No.1. The filtrate was left to evaporate inside an incubator for two days to get about 2 grams of dry extract.
Test microorganisms
Referenced bacterial and yeast strains were obtained from the Department of Pharmaceutics, Unaizah College of Pharmacy, Qassim University, which included four Gram-positive, namely Staphylococcus aureus ATCC 25923, Streptococcus pneumoniae ATCC 49619, Staphylococcus saprophyticus ATCC 43867, Bacillus cereus ATCC 10876; four Gram-negative bacteria, namely Escherichia coli ATCC 10535, Shigella flexneri ATCC 12022, Proteus vulgaris ATCC 6380, Salmonella Typhimurium ATCC 14028; and one yeast, namely Candida albicans ATCC 10231.
Inoculum preparation
Microbial cultures were identified microscopically and biochemically with routine tests, then sub-cultured in sterile Petri-dishes containing nutrient broth for bacteria or sabouraud dextrose agar for yeast and incubated for 24 hours for bacteria or 48 hours for yeast at 37 °C. Prior to the antibacterial experiment, the working microbial suspension was adjusted to be equivalent to McFarland standard, approximately 108 CFU/ml of bacteria or 106 CFU/ml of yeast.
Disc-diffusion assay
The dried methanol extract of lichen was dissolved in the same solvent (80% methanol) to a final concentration of 300 mg/ml. The antimicrobial potential was evaluated using the disc-diffusion method [8], with minor modification. 100 µl from the working microbial suspensions (previously adjusted) was poured over plates containing sterile Mueller-Hinton agar for bacteria or sabouraud dextrose agar for yeast. Sterile blank paper discs cut from Whatman No.1 paper (6 mm in diameter) were impregnated with 10 µl of lichen methanolic extract at the concentration of 300 mg/ml (3000 µg/disc) and put on the inoculated agar medium. The pre-experimental test showed no effect of 80% methanol on microorganisms. Also, discs impregnated with 10 µl of antibiotics (chloramphenicol 2.5 mg/ml for bacteria or clotrimazole 5mg/ml for fungi) were used as a positive control. Cultured plates were incubated overnight of bacteria or 48 hours for yeast at 37oC. Zone of inhibition (ZI) was measured and mean of two replicates was calculated.
MIC, MBC and MFC assay
The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC), were carried out for microorganisms susceptible to the lichen extract, using the method previously reported elsewhere [9], with minor modification. For the MIC, 8 tubes (size 5 ml) were loaded with 1 ml of liquid medium (nutrient broth for bacteria or sabouraud dextrose broth for yeast), sealed and autoclaved. Then, 1 ml of lichen extract (100mg/ml) were poured in the 1st tube and serial two-fold dilutions were made till the 6th tube, to get dilutions from 100 to 3.125 mg/ml. 100 µl from the adjusted microbial suspension was poured to each tube. The other two tubes were used as positive control (inoculated tubes with chloramphenicol or clotrimazole) and negative control (inoculated tubes with solvent only). All tubes were incubated for at 37oC for 24 hours (for bacteria) and 48 hours (for yeast). The lowest concentration which showed no visible turbidity (no growth) compared with the negative control tube was taken as MIC. To determine the MBC values, a portion of liquid (50 µl) from all tubes that showed no growth (no obvious turbidity) were poured on a sterile Mueller-Hinton Petri-dishes, incubated and inspected for microbial growth. MBC (for bacteria) and MFC (for fungi). MBC and MFC values were the highest dilution that exhibited no single microbial colony on agar medium.
Statistical analysis
Data were expressed as mean ± standard error of means and statistical analyses were carried out using the SPSS package (SPSS for Windows, version 14) (Chicago, IL, USA).
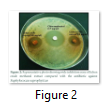
The results of the antimicrobial activity of the methanol extract of Platismatia glauca using disc diffusion method are shown in (Table 1). This lichen showed wide-spectrum antimicrobial properties against all tested microorganisms. The most susceptible bacterium was Staphylococcus saprophyticus, which recorded 18.5±1.0 mm zone of inhibition (Figure 2), followed by Staphylococcus aureus (14.5±0.5 mm), Shigella flexneri (12.5±1.5 mm), Streptococcus pneumoniae (12.0±1.0 mm), Proteus vulgaris (11.5±0.5 mm), Salmonella Typhimurium (11.5±0.5 mm), Bacillus cereus (11.0±1.0 mm) and Escherichia coli (11.0±0.0 mm), respectively. It also showed high antifungal activity against Candida albicans which was 22.5±0.5 mm. These results were also compared with a standard antibacterial drug (Chloramphenicol 2.5mg/ml) and a standard antifungal drug (Clotrimazole 5 mg/ml) (Figure 3).
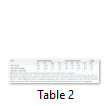
The results of the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) of the methanol extract of Platismatia glauca against the tested microorganisms are represented in (Table 2). Based on the MIC results, the lichen extract was bacteriostatic against one bacterium at low concentration of the extract, which was 3.125 mg/ml for Staphylococcus saprophyticus. However, the methanol extract was bacteriostatic against the majority of bacterial strains at 6.25 mg/ml (6 out of 9 bacterial strains) and it was bacteriostatic only against Escherichia coli at 12.5 mg/ml. As well, it was fungi-static against the yeast (Candida albicans) at 6.25 mg/ml. The MBC and MFC values revealed that the methanol extract of this lichen (Platismatia glauca) was bactericidal or fungicidal against all tested microorganisms at 12.5 mg/ml, except with Staphylococcus saprophyticus and Proteus vulgaris, where the bactericidal effects were recorded at 6.25 mg/ml.
Figures & Tables
In the current study, the methanol extract of Platismatia glauca, a lichen widely consumed as a spice showed a remarkable antibacterial activity against all tested Gram-positive and Gram-negative bacteria, Furthermore, the extract exhibited high anti-candidal activity. According to Philip et al. [10], natural product extracts that recorded inhibition zones with disc diffusion assay above 10 mm is considered as good antimicrobial activity. This supports our findings, where the current results were ranging from 11.0±0.0 to 22.5±0.5 mm. The most pronounced activity was against Candida albicans (22.5±0.5 mm), which is considered as an opportunistic pathogenic yeast, infected skin, oral cavity, esophagus, vascular system, gastrointestinal tract and vagina of human being and possesses serious virulence factors threaten the health and life of people particularly the immunocompromised [11]. Overall, some studies supporting the current results on Platismatia glauca, Mitrović et al. [12] cited that lichen species Platismatia glauca showed varying considerable degrees of antimicrobial and antibiofilm activity against 11 bacterial and 9 fungal strains. Gulluce et al. [13] stated that Platismatia glauca represented antimicrobial activity against different bacterial and fungal strains, namely Bacillus macerans A199, Bacillus subtilis ATCC 6633, Bacillus subtilis A57, Clavibacter michiganense A227, Sclorotinia sclerotiorum and Trichophyton rubrum. Moreover, many other lichen species revealed antimicrobial properties. Sweidan et al. [14] published that some compounds such as (+)-Roccellic acid (R), Demethyl barbatic acid (D) and Psoromic acid (P) extracted from various lichen species, namely Ramalina siliquosa, Letharia vulpine, Roccella fuciformis and Roccella phycopsis possesses significant antibacterial activity against oral bacteria, namely Streptococcus gordonii and Porphyromonas gingivalis. The study also showed high antibacterial activity against the gram-positive Staphylococcus saprophyticus which is a pathogen widely associated with urinary tract infections, particularly in women [15]. Since both microorganisms (Candida albicans and Staphylococcus saprophyticus are causative agents of urinary tract infections (UTI), lichen thalli of Platismatia glauca could be a promising source for a drug against UTI. The results of the disc diffusion test were supported with MIC, MBC and MFC tests which confirmed the wide-spectrum antimicrobial properties of this lichen species. As represented in (Table 2), the MBC/MIC ratios were ranging from 1 to 2. It was reported that the extract is considered to have bactericidal characteristics when the ratio of MBC/MIC is ≤ 4 and assumed as bacteriostatic if the ratio of MBC/MIC is >4 [16]. Accordingly, the methanol extract of Platismatia glauca thalli has some bactericidal properties, indicating that it can be used as an antimicrobial drug. Finally, the antimicrobial activity detected in this investigation is attributed to the secondary metabolites secreted by lichens. Gulluce et al. [13] noticed that the total phenolic constituents of extracts from the lichen (Platismatia glauca) were 1.1% (w/w), implying that the antimicrobial activity is related to these polar phenolics. Therefore, further future studies using different polar solvents are recommended in order to isolate these antimicrobial constituents and it should have supported by toxicological, pharmacological and in vivo studies.
The lichen thalli of Platismatia glauca, showed remarkable antimicrobial activity. Accordingly, this lichen represents a powerful source of new antimicrobial molecules for drug industries and food preservation. The study is also validating its utilization as an antimicrobial drug in ancient traditional medicine. Further studies are recommended for isolation of pure active components in this lichen species.
Acknowledgement
Thanks to Dr. Kamal A. Qureshi, for allowing the author to take inoculums of the ATTC@ referenced microbialstrains available at the Department of Pharmaceutics, Unaizah College of Pharmacy, Qassim University, Saudi Arabia.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Mol ML, Snoeck N, Maeseneire SL, Soetaert WK. Hidden antibiotics: Where to uncover?. Biotechnology Advances, (2018); 36(8): 2201-2218.
- Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food Control, (2014); 46: 412-429.
- Škrinjar MM, Nemet NT. Antimicrobial effects of spices and herbs essential oils. Acta period Techno, (2009); 40: 195-209.
- Padhi S, Tayung K. In vitro antimicrobial potentials of endolichenic fungi isolated from thalli of Parmelia lichen against some human pathogens. Beni-Suef University Journal of Basic and Applied Sciences, (2015); 4: 299-306.
- Mitrović T, Stamenković S, Cvetković V, Nikolić M, Tošić S, Stojičić D. Lichens as source of versatile bioactive compounds. Biologica Nyssana, (2011); 2(1): 1-6.
- Gomez-Serranillos MP, Fernandez-Moriano C, Gonzalez-Burgos E, Pradeep Kumar Divakar PK, Crespo A. Parmeliaceae family: phytochemistry, pharmacological potential and phylogenetic features. RSC Advances, (2014); 4: 59017-59047.
- Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA. The lichen of Great Britain and Ireland. 2nd Edn. (2009). British Lichen Society, UK.
- Karaman I, Sahin F, Güllüce M, Ögütçü H, ¸Sengül M, Adıgüzel A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. Journal of Ethnopharmacology, (2003); 85: 231-235.
- Abdallah EM, Qureshi KA, Ali AMH, Elhassan GO. Evaluation of some biological properties of Saussurea costus crude root extract. Bioscience Biotechnology Research Communication, (2017); 10(4): 601-611.
- Philip K, Malek SNA, Sani W, Shin SK, Kumar S, Lai HS, Serm LG, Rahman SNSA. Antimicrobial activity of some medicinal plants from Malaysia. American Journal of Appllied Sciences, (2009); 6: 1047-1058.
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends in Microbiology, (2001); 9(7):327-335.
- Mitrović T, Stamenković S, Cvetković V, Radulović N, Mladenović M, Stanković M, Topuzović M, Radojević I, Stefanović O, Vasić S, Čomić L. Platismatia glauca and Pseudevernia furfuracea lichens as sources of antioxidant, antimicrobial and antibiofilm agents. EXCLI Journal, (2014); 13: 938-953.
- Gulluce M, Aslan A, Sokmen M, Sahin F, Adiguzel A, Agar G, Sokmen A. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine, (2006);13: 515–521.
- Sweidana A, Chollet-Krugler M, Sauvager A, Weghe P, Chokrc A, Bonnaure-Mallet M, Tomasi S, Bousarghin L. Antibacterial activities of natural lichen compounds against Streptococcus gordonii and Porphyromonas gingivalis. Fitoterapia, (2017); 121: 164–169.
- Raz R, Colodner R, Kunin CM. Who Are You—Staphylococcus saprophyticus?. Clinical Infectious Diseases, (2005); 40(6): 896–898.
- Djeussi DE, Noumedem JA, Seukep JA, Fankam AG, Voukeng IK, Tankeo SB, Nkuete AH, Kuete V. Antibacterial activities of selected edible plants extract against multidrug-resistant Gram-negative bacteria. BMC Complementary and Alternative Medicine, (2013); 13: 164.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0