Full Length Research Article
Toxicopathological effects of endosulfan in female Japanese Quails (Coturnix japonica)
Tahir Hussain1, Muhammad Kashif Saleemi1*, Muhammad Zargham Khan1, Ahrar Khan1,2, Rao Zahid Abbas1, Muhammad Qamar Bilal3, Farrah Deeba1, Hamid Irshad4, Zahida Fatima4, Farhan Afzal5, Umar Farooq6, Muhammad Moazam Jalees7
Adv. life sci., vol. 7, no. 2, pp. 72-78, February 2020
*- Corresponding Author: Muhammad Kashif Saleemi (Email: drkashif313@gamil.com)
Authors' Affiliations
2. Shandong Vocational Animal Science and Veterinary College, Weifang, 261061, China
3. Institute of Animal and Dairy Sciences, University of Agriculture Faisalabad Pakistan
4. PARC National Agricultural Research Center, Islamabad, Pakistan
5. Poultry Research Institute, Murree Road Rawalpindi, Pakistan
6. Subcampus Toba Tek Singh, University of Agriculture Faisalabad Pakistan
7. Department of Microbiology Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: The current study was planned to investigate the toxico-pathological effects of endosulfan in female Japanese quails.
Methods: A total of 120 quail of 4 weeks old were divided into six equal groups (A-F) and administered endosulfan in feed at dose rate of 0, 5, 25, 50, 100, and 500 mg/kg feed, respectively for 90 days. Parameters studied included clinical signs, feed intake, body weight and mortality. Hematology, serum biochemistry, hatchability and fertility were also determined. Gross and microscopic changes on different organs were recorded.
Results: The quails of the group B did not show any clinical signs and had significantly lower values of feed intake, testes relative weight and leukocyte number than those of the control group A. The quails of group C and D had mild depression while those of the group E and F showed nervous excitation following ingestion of endosulfan. There was a dose related delay in onset of crowing, appearance of foamy material in the droppings. The feed intake, erythrocyte and leukocyte counts, hematocrit values, and serum total proteins of endosulfan fed quails were significantly (p < 0.05) lower than that of the group A. The total egg production in groups A, B and C was significantly higher from group D, E and F. The hatchability in group A and B was significantly higher from groups C, D, E and F. The difference of dead in shell % and early dead among different groups was nonsignificant. Infertile egg percentage was significantly higher in group E compared with all other groups except group F. The necrotic changes were observed in all parts of oviduct in high dose groups, similarly necrotic changes and vacuolar degeneration was observed in hepatic parenchyma in high dose groups D-F.
Conclusion: It may be concluded that endosulfan leads to dose dependent changes in the quails.
Keywords: Body weight; Coturnix japonica; Endosulfan; Haematological values; Histopathology
Pesticides, heavy metals and mycotoxins contamination is a global issue effecting badly to our environment [1,2,6,21]. Endosulfan, a chlorinated hydrocarbon pesticide of the cyclodiene subgroup is used to protect the crops from a wide variety of insects and mites. The chemical formula for endosulfan is C9H6Cl6O3S. It is effective against a wide range of insects and certain mites on cotton, cereals, grains, coffee, fruits, oil seeds, potatoes, tea and vegetables. Technical endosulfan is made up of a mixture of alpha- and beta-isomers. In Pakistan, endosulfan is available in agricultural market with trade names like Thiodan, Hektionex, Technofan, Fentox etc.
Endosulfan residues persist in the soil with an average half-life of 55-277 days [26]. This period of half-life develops a toxicological hazard for nontarget species including wild birds, free range chicken and livestock kept by the farmers. Endosulfan gained entry into the body through oral route, inhalation or directly through skin. It causes endocrine disruption, can either imitate or block hormones like estrogen, testosterone and thyroid hormones. These are extremely powerful chemicals normally present in the body in micro quantities. Therefore, an even very low level of the pesticides that imitate or block the function of these hormones significantly alters their effects [12,13,16,22,25]. In human beings endocrine disrupters act more subtly, resulting in birth defects, infertility, and learning disabilities. Endosulfan is also toxic to reproductive system and its reproductive toxicity and teratogenic effects have been reported in female and male mice and rats. In acute toxicity it causes stimulation of nervous system, dyspnea, cyanosis, tremors, and convulsions [20]. In chronic toxicity there is reduced growth, liver enlargement, and change in blood and organ chemistry [11].
Natural cases of endosulfan toxicities as well as its experimental production have been reported in different species including rats, mice, rabbits, fish, chicken, cow, human etc. [22]. Free-range poultry in villages and wild birds’ population is exposed to endosulfan by feeding on the contaminated grains or other plant components as well as by eating contaminated insects died of endosulfan application. Toxic effects of endosulfan are well documented in different species including rats, however, information regarding toxic effects and morphological changes induced in different organs particularly female reproductive system in avian species is not available in the accessible literature. With these intensions this project has been designed to study the endosulfan toxicity in Japanese quails with particular reference to female reproductive system. The results of this study would be helpful to diagnose and differentiate the clinical syndromes of obscure etiologies in these species.
Birds’ management
A total of 120 female Japanese quails (Coturnix japonica) at the age of 25 days were purchased from a local farm. Birds were kept in metal wire cages at ambient temperature in a poultry house. The birds were fed on commercial broiler starter feed containing 21 % total protein (Aniâ Feeds, Gujranwala) as basal feed. All the birds were given feed and water ad libitum.
Experimental design
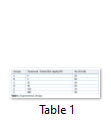
The birds were divided randomly into six equal groups A-F having 20 birds in each group. These groups were kept in separate cages. After one week of acclimatization, the birds were administered endosulfan (Thiodanâ 35 EC, 32.9 % w/w endosulfan and 67.1% solvent w/w, Aventis, Pakistan) incorporated in the feed at different levels (table 1).
The duration of the experiment was 90 days. Five birds were slaughtered on day 15 and 30 of the experiment from each group and remaining birds at the end of experiment day 90.
Parameters studied
Clinical signs and behavioral alterations twice daily. A subjective score range 0-4 was selected for clinical signs. For no signs score was 0 and most severe sign the score was 4. These included attraction towards feed and water, foaminess in droppings etc. Feed intake on daily basis, bodyweights weekly, mortality on daily and % mortality was calculated.
Hematology and serum biochemistry
The six birds from each group were sacrificed on 15th, 30th day and remaining all at the end of experiment of experiment to collect blood for hematobiochemical analysis. Microhematocrit method [5] was used to determine hematocrit and hemoglobin concentration was measured by cyanmethemoglobin method. Total protein was measured by Biuret method and albumin by Bromocresol Green Dye Binding method using commercially available colorimetric kits of Merck Company following instructional manual.
Hatchability parameters
Eggs were collected on daily basis and hatch was set weekly in automatic incubator. After every hatch, hatch analysis was conducted and record of the total eggs set, hatched, early dead, dead in shell and infertile eggs were collected.
Gross and microscopic pathology
On each slaughtering, organs were collected, weighed and examined for gross pathology liver, kidneys and oviduct tissues were fixed in 10% neutral buffered formalin for histopathology [16].
Statistical analysis
The data thus obtained were analyzed statistically by analysis of variance test (ANOVA) and group means were compared by Duncan’s multiple range test. The level of the significance was p ≤ 0.05.
Clinical signs and behavioral alterations
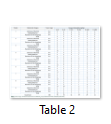
The results of clinical signs and behavioral alterations have been presented in table 2.
Feed intake
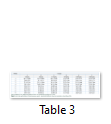
Feed intake of Japanese quails in different groups has been presented in Table 3. In 1st week of the experiment the feed intake of group E was significantly higher from group C and D. In second week of experiment the feed intake of group D was significantly higher from all other groups. In 3rd week the feed intake of group A was significantly higher from group C and D and nonsignificantly higher than group B, E and F. In 4th week the feed intake of group A was significantly higher from group C and D and nonsignificantly higher than group B, E and F. In 5th week the feed intake of group A and B was significantly higher from all other groups. In 6th week the feed intake of group A and B was significantly higher from all other groups. In 7th week feed intake of group A was significantly higher from all other groups. Group A was significantly higher in feed intake from all other groups. In last week of the experiment the feed intake of group A and B was significantly higher from all other groups.
Body weight
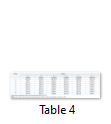
Body weights of the birds in different groups have been presented in the Table 4. Difference in body weight among different groups was nonsignificant throughout the length of the experiment.
Effect of endosulfan on relative organ weights
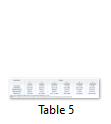
The difference in weight of ovary among different groups was nonsignificant up to 30 days (table 5). From day 30 to 90 the ovary weight of group A was significantly higher from group C, D, E and F. The difference in weight of oviduct among different groups was nonsignificant up to 30 days. From day 30 to 90 the oviduct weight of group A to D was significantly higher from groups E and F but nonsignificant difference among each other. The difference in weight of kidney among different groups was nonsignificant throughout the length of experiment. The difference in weight of liver among different groups was nonsignificant up to day 60. From day 30 to 90 the liver weight of group C was significantly higher from groups A, B and F but it was nonsignificantly higher from group D and E. The difference in weight of heart among different groups was nonsignificant throughout the length of experiment. The difference in weight of spleen among different groups was nonsignificant throughout the length of experiment.
Effect of endosulfan on hematological parameters
There was a significant difference in erythrocyte count noted among control group and group D, E and F on day 15, 30 and 90 of experiment as shown in Fig. 1. Leukocytes count of group A was significantly higher from all other groups on day 30. From day 30 to 90 leukocytes count was significantly higher in group A and B from group D and F. The difference between group A and B was nonsignificant. There was a significant difference in hematocrit value observed among control group and group E and F on day 15. The group A and B were significantly higher in hematocrit value from all other groups. From day 30 to 90 group A had significantly higher value from group E and F, while nonsignificantly higher from groups B, C and D. There was a significant difference observed in hemoglobin concentration among control group and all other groups on day 15 of the experiment. However, nonsignificant difference was observed on day 30 and 90 of experiment as shown in fig. 1
Effects of endosulfan on serum biochemistry
Serum biochemistry
The difference in serum total protein values among different groups throughout the experiment were nonsignificant (Fig. 1). The difference in serum albumin values among different groups throughout the experiment was nonsignificant.
Effect of endosulfan on egg production and hatchability parameters
Different parameters related hatchability were shown in table 5.
Total Egg production:
The total egg production in groups A, B and C was significantly higher from group D, E and F. The difference in the total egg production among group A, B and C was nonsignificant.
Hatchability (%): The hatchability in group A and B was significantly higher from group C, D, E and F.
Dead in Shell (%): The difference of dead in shell % among different groups was nonsignificant through the length of experiment.
Early dead (%): The difference of early dead in shell % among different groups was nonsignificant throughout the length of experiment.
Infertile (%): Infertile egg percentage was significantly higher in group E compared with all other groups except group F.
Gross pathology
No gross lesions were observed in all the groups that were administered endosulfan at different dose levels.
Microscopic pathology
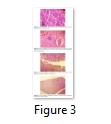
Oviductal size was normal in group A and B (Fig. 3 c). The tubular glands atrophy and patent lumen with decreased mucosal folds height in magnum and isthmus with epithelium only in magnum were noted in oviduct of quails treated with endosulfan from C-F groups (Fig. 3 d). At few places degenerated nuclei were showing necrotic changes. There were also cellular infiltration in stroma of folds of magnum showing inflammatory response there. In the liver in group D, E and F necrotic changes were present in the hepatocytes and biliary hyperplasia was also present in the portal area (Fig. 3 b). No such changes were present in the group A and B (Fig. 3 a). In kidneys in the group D, E and F tubular necrosis was present.
Figures & Tables
Food security is still a significant Millennium Development Goal in most of the developing countries [29]. Endosulfan is an important member of organochlorine group of pesticides. Endosulfan is persistent in the soil and its average field half life is 55-277 days [26]. Residues of endosulfan present in soil environment, on fruits and on different plant parts pose a high risk to the domestic and wildlife species living in agricultural areas. Domestic and wild birds may also be intoxicated by eating dead insects contaminated by endosulfan.
Reports describe the acute [20] and chronic [11] effects of endosulfan in different species. However a little information is available regarding endosulfan toxicity in domestic and wild birds. This study describes the pathological changes, experimentally induced in female Japanese quails by endosulfan mixed in their feed.
Female Japanese quail (Coturnix japonica) is a domesticated bird which grows rapidly and reaches at puberty in a short period. It therefore acts as model bird for experimental studies in avian species. The results obtained from experiments from female Japanese quails may be extrapolated, domesticated birds like chicken as well as wild birds.
Neurological excitement was an important sign observed in endosulfan treated quails. The nervous signs in the present study appeared in quails of given 25 mg endosulfan/kg feed gradually became more prominent with increase in dose level, highest in F group that was given 500 mg endosulfan/kg feed. This indicates dose related neurological excitement due to endosulfan fed to them. Neurological excitement characterized by excitability, and transient torticollis. Nervous excitement following administration of endosulfan have been reported in pigeons [3], human beings, [7] and rats [10].
A significant reduction in feed intake was observed in birds fed endosulfan 25mg / kg feed or above. Feed intake was dose related, decrease being reciprocal to endosulfan intake by the birds. A decrease in feed intake has also been reported by [8] in rats. It appears that apart from other pathological manifestations, endosulfan also induced decreased feed intake.
Many authors have reported the nephrotoxic effects of endosulfan. Earlier, studies reported the highest concentrations of alpha and beta isomers of racemic endosulfan in rats [4,22]. The toxicity of endosulfan on kidneys of male rats in relation to drug metabolizing enzymes has already been established [27]. Histological changes have been reported earlier in which an acute tubular necrosis following endosulfan insecticide poisoning was observed [18]. A difference in species LD50 has also been reported in rats after oral administration [28] from 18 to 160 mg/kg. The reported 5-day dietary LC50 is 2906 ppm in Japanese quail [14]. In fish species, the reported 96-hour LC50 value was 1.5ug/L in rainbow trout [17].
Hematological parameters responded to endosulfan toxicity by a significant decrease in erythrocyte and leukocyte counts along with decrease in hematocrit level at higher doses (100mg endosulfan/kg feed and above). These observations suggest inhibitory effect of endosulfan on hematopoietic organs. However, research reported nonsignificant difference on complete blood count induced by endosulfan administration [30].
A decrease in serum total proteins and albumin has been observed in the lateral stages of endosulfan toxicity. This reduction has also been reported in literature [11]. This decrease could be due to degenerative changes produced by endosulfan in the liver.
A significant degenerative effect of endosulfan was observed in female Japanese quails. This degenerative effect was characterized by delayed and low egg production in groups that were administered endosulfan. A gradual delay in onset of puberty, low egg production, more number of unhatched eggs and increase in number of infertile eggs with increase in endosulfan level in feed of quails suggests a dose related increase in degenerative effects on female reproductive system.
In present study, endosulfan also exerts injurious effects on female reproductive system. Reduction in weight of ovaries and oviduct, a smaller number of eggs early embryonic deaths have been observed in the quails administered endosulfan in feed in the present study. Endosulfan induced gonadotoxicity has been reported by many authors. Michael reported the reproductive effects in birds exposed to pesticides and industrial chemicals [19]. Effects on breeding adults as well as developmental effects on embryos were studied. Papov studied embryo and genotoxic effects of endosulfan on rat embryo [24]. Dalsenter et al., reported reproductive effects of endosulfan on male off-springs of rats exposed during pregnancy and lactation. Microscopic picture of reproductive organs revealed pyknotic nuclei, foamy cytoplasm with excessive vacuolation, decreased size of glands. These histomorphological changes were also documented [23]. This decrease could be due to degenerative changes produced by endosulfan in the reproductive organs.
Authors' Contribution
TH, MKS, MMJ, UF and MZK plan the study and executed the experiment and performed lab work, RZ, AK, HI helped in the data analysis and MZK, TH, MKS, MJ, FD, QB, FA and ZF were involved in manuscript preparation.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Ahmad KR, Shehry K, Raees K et al., Adverse effects of cypermethrin on the chick (Galus domesticus) development are reversed by co-treatment with vitamin E and olive oil. Pakistan Veterinary Journal (2018); 38: 46-50.
- AL-Ruwaili M, Alkhalaileh NI, Herzallah SM et al. Reduction of aflatoxin b1 residues in meat and organs of broiler chickens by lactic acid bacteria. Pakistan Veterinary Journal (2018); 38(3): 325-328.
- Anand M, Agrawal AK, Gopal K et al. Endosulfan and cholinergic (muscarinic) transmission: effect on electroencephalograms and [3H] quinuclidinyl benzilate in pigeon brain. Environmental Research (1986); 40:421-6.
- Ansari RA, MK Siddiqui, PK Gupta. Toxicity of endosulfan: distribution of alpha- and beta-isomers of racemic endosulfan following oral administration in rats. Toxicology Letters, (1984); 21(1):29-33.
- Benjamin MM. Outline of veterinary clinical pathology (1978), 2nd Ed. The Iowa University press, USA.
- Butt SL, Saleemi MK, Khan MZ et al. Cadmium toxicity in female Japanese quail (Coturnix japonica) and its diminution with silymarin. Pakistan Veterinary Journal, (2018); 38: 249-255.
- Cable GG, Doherty S. Acute carbamate and organochlorine toxicity causing convulsions in an agricultural pilot: a case report. Aviat. Space Environmental Medicine, (1999); 70(1):68-72.
- Chitra KC, Latchoumycandane C, Mathur PP. Chronic effects of endosulfan on the testicular functions of rat. Asian Journal of Andrology, (1999); 1:203-206.
- Dalsenter PR, Dallegrave E, Mello JR, Langeloh A et al. Reproductive effects of endosulfan on male offspring of rats exposed during pregnancy and lactation. Human and Experimental Toxicology, (1999); 18(9):583-589.
- Dikshith DS, Raizada RB, Kumar SN et al., Effect of repeated dermal application of endosulfan to rats. Veterinary and Human Toxicology, (1988); 30(3):219-224.
- Gill TS, Pande J, Tewari H. Effects of endosulfan on the blood and organ chemistry of freshwater fish, Barbus conchonius Hamilton. Ecotoxicology and Environmental Safety, (1991); 21(1):80-91.
- Gul ST, Khan A, Farooq M, Niaz S et al., Effect of Sub Lethal Doses of Thiamethoxam (A Pesticide) on Hemato-Biochemical Values in Cockerels. Pakistan Veterinary Journal, (2017); 37(2): 135-138.
- Gul ST, Khan A , Ahmad M, Anwar MF et al., Effect of sub-lethal doses of thiamethoxam (a neonicotinoid) on hemato-biochemical parameters in broiler chicks. Toxin Reviews, (2018); 37(2): 144–148.
- Hill, EF, Camardese MB. Lethal Dietary Toxicities of Environmental Contaminants to Coturnix, Technical Report Number 2. U.S. Department of Interior, Fish and Wildlife Service, Washington, (1986); DC. 6-55.
- Hussain R, Ali F, Rafique A, Ghaffar A et al., Exposure to sub-acute concentrations of glyphosate induce clinico-hematological, serum biochemical and genotoxic damage in adult cockerels. Pakistan Veterinary Journal, (2019); 39(2): 181-186.
- Hussain R, Ghaffar A, Ali HM, Abbas RZ et al., Analysis of different toxic impacts of Fipronil on growth, hemato-biochemistry, protoplasm and reproduction in adult cockerels, Toxin Reviews, (2018); 37:4, 294-303
- Johnson WW, Finley MT. Handbook of Acute Toxicity of Chemicals to Fish and Aquatic Invertebrates, Resource Publication 137. U.S. Department of Interior, Fish and Wildlife Service, Washington, (1980); DC, 6-56.
- Lo RS, Chan JC, Cockram CS, Lai FM. Acute tubular necrosis following endosulfan insecticide poisoning. Journal of Toxicology Clinical Toxicology, (1995); 33(1):67-9.
- Michael Fry D. Reproductive Effects in Birds Exposed to Pesticides and Industrial Chemicals. Environmental Health Perspectives, (1995); 103(7).
- Naqvi SM Vaishnavi C. Bioaccumulative potential and toxicity of endosulfan insecticide to non-target animals. Comparative Biochemistry and Physiology, (1993); 105(3):347-361.
- Naseem MN, Saleemi MK, Abbas RZ et al., Hematological and serum biochemical effects of aflatoxin B1 intoxication in broilers experimentally infected with fowl adenovirus-4 (FAdV-4). Pakistan Veterinary Journal,(2018); 38(2): 209-213
- Qamar AB, Khan MZ, Khan A, Saleemi MK, Javed I. Endosulfan (Thiodan@ EC 35) induced toxicopathological effects in male Japanese quails (Coturnix japonica). Journal of National Science Foundation of Sri Lanka, (2012); 40: 97-105.
- Pandey AC. Impact of endosulfan (thiodan) EC 35 on behavior and dynamics of oocyte development in the teleostean fish, Colisa (Trichogaster) fasciatus. Ecotoxicology and. Environmental Safety, (1988); 15:221-225.
- Papov. Embryo and genotoxic effects of two endosulfan forms in the culture of rat and mouse pre- and postimplantation embryos. Ontogenez, (1998); 29: 104-112.
- Riaz A, Ulhaq M, Khan IA, Khan A et al., Chlorpyrifos induced dermal toxicity in albino rabbits. Pakistan Veterinary Journal, (2018) 38(1): 91-95.
- Shalini-Singh, PD, Kumar S. Biodegradation of alpha and beta isomers of endosulfan and endosulfan sulphate in Indian soils. Journal of Environmental Sciences and Health, (2000); 5(3):337-346.
- Singh, SK and Pandey RS. Toxicity of endosulfan on kidney of male rats in relation to drug metabolizing enzymes and microsomal lipid peroxidation. Indian Journal of Experimental Biology, (1989); 27(8):725-8.
- Smith, AG. Chlorinated Hydrocarbon Insecticides. In Handbook of Pesticide Toxicology. Hayes, WJ, Jr and Laws, ER, Jr, Eds. Academic Press Inc., New York, (1991); 6-3.
- Subhani Z, Shahid M, Hussain F and Khan JA, Efficacy of Chlorella pyrenoidosa to ameliorate the hepatotoxic effects of Aflatoxin B1 in broiler chickens. Pakistan Veterinary Journal, (2018) 38: 13-18
- Venkateswarlu, K, Suryarao K, Srinivas V et al., Endosulfan poisoning–a clinical profile. J Assoc. Physicians India, (2000); 8(3):323-5.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0