Full Length Research Article
Effect of Serine on Growth and Biochemical Constituents of Zea mays L., Triticum aestivum L., and Abelmoschus esculentus L. under Arsenic Toxicity
Sabrina Shahid1, Fayaz Asad2*, Fida Hussain3, Tabassum Yaseen2, Naveen Dilawar4, Imtiaz Ahmad2, Sharipova Vasila5
Adv. life sci., vol. 10, no. 3, pp. 398-405, September 2023
*- Corresponding Author: Fayaz Asad (fayaz.asad79@gmail.com)
Authors' Affiliations
2. Department of Botany, Bacha Khan University Charsadda, KP – Pakistan
3. Department of Botany, Islamia College University Peshawar, KP – Pakistan
4. Department of Botany, Abdul Wali Khan University Mardan, KP – Pakistan
5. Institute of Botany, Academy of Sciences Republic of Uzbekistan, Tashkend Durmon Yuli – Uzbekistan
[Date Received: 24/01/2023; Date Revised: 14/07/2023; Date Published: 30/09/2023]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Various human activities, such as industrialization, modern farming methods, and mining increase the concentration of heavy metals in air, water and soil. Heavy metal poisoning of soil results in a number of environmental issues and has deleterious effects on both plants and animals. Therefore, the purpose of this study was to investigate the effects of Arsenite (As) and As+ Serine (Ser) on growth and biochemical components in the early growth stages of Abelmoschus esculentus (L.) Moench, Triticum aestivum L., and Zea mays L. (selected crops).
Methods: Pot experiments were carried out at completely random manner, with 10-12 seeds grown in each pot with three replicates. Seeds and seedlings in pots treated with different concentrations of As and As+Ser. After a 21-days of germination period, we gathered the growth-related parameters (root number, root length, shoot length, and leaf number) and conducted a biochemical analysis.
Results: The growth of selected plants was adversely impacted by Arsenic stress, whereas the detrimental impact was minimal after treatments with Serine. Compression of the selected crops showed that Abelmoschus esculentus L. had the most detrimental impact on agronomic parameters. Biochemical constituents such Chlorophyll “a” “b”, Total-chlorophyll (Photosynthetic pigments), protein and carotenoid contents formation were reduced at individual treatments of As (25, 50, 75 and 100pmm) compared to As+Ser and control treatment, while the proline contents were increased considerably at treatment 100 ppm (As) of the selected crops.
Conclusion: The results showed that As had a greater negative impact on growth and biochemical constituents, whereas Ser had a reduced adverse impact on selected crops. Abelmoschus esculentus L. had a higher sensitivity compared to other selected crops.
Keywords: Arsenite (As) stress; Plant growth regulator; Plant growth; Abiotic stress
As is abundant in the earth's crust, ranking 20th overall, 14th in seawater, and 12th in terms of abundance in human tissue [1]. Many natural processes contribute to the militarization of As in the environment, including weathering reactions, biochemical pathways, human activity processes, and volcanic emissions [2]. Because the earth’s crust has a high As content, it can easily leach into underground water [3]. Arsenic is a large natural toxin, and the harm it does is becoming increasingly concerning for a number of factors, including ecological, transformational, healthy, and ecological ones [4]. As poisoning of the environment is harmful to plant, animal, and human health [5]. As may infiltrate terrestrial and aquatic habitats as a result of manmade activity as well as natural creation [6]. Concern has been raised about the persistence of As in soil and its toxicity to both plants and animals [7]. Plants can come into contact with As in a variety of ways. The most significant one is most likely from irrigation of crops by As contaminated groundwater [8]. Irrigation is mostly carried out during the dry season for the production of Boro rice. Tube wells have been widely used in Pakistan to irrigate millions of hectors of agricultural land [9], considerably increasing the nation's output of food grains. As's content in crops is expected to rise with prolonged irrigation with groundwater that is polluted with the metal.
Most plants are poisoned by As in greater concentrations. As-induced phytotoxicity disrupts metabolic pathways and prevents plant growth and development [10]. When crops are exposed to high quantities of As, either in soil or in solution culture, they display toxicological symptoms such as inhibition of seed germination [11,12]; decrease in plant height [2,13-15]; reduction of root development [16-18]; lower fruit and grain yield [19]; reduction in shoot growth [20]; and, rarely, cussed death [21-23].
As impact on agronomic parameters and photosynthetic pigments, which form the basis of the biochemical system in plants, is not well understood. As almost all of the negative physiological and agronomical effects of As are connected to the fundamental photochemical process of photosynthesis in plants. Therefore, it is crucial to measure the influence of As on the growth and major photosynthetic pigments Chlorophyll-a, 'b', and biochemical constituents such as protein, carotenoid, and proline in Abelmoschus esculentus (L.) Moench, Triticum aestivum L., and Zea mays L., under the Ser treatments. The goal of the current study was to determine how soil As concentrations affected the various crop growth phases and chlorophyll biochemical constituents of three frequently grown species: Triticum aestivum L., Zea mays L., and Abelmoschus esculentus (L.) Moench in Pakistan.
The Khyber Pakhtunkhwa agricultural research center provided the Zea mays L., Triticum aestivum L., and Abelmoschus esculentus (L.) Moench seeds for this study. The pot experiments were conducted in greenhouse of Bacha Khan University Charsadda. It has 126 pots and three replicates for each dose/application. The soil was collected from agricultural field of District Charsadda and subjected to below different As and Ser concentrations;
Set 1
Control (only distilled)
Set 2
As = 25, 50, 75 and 100ppm
Set 3
As × Ser = 50× 25, 50×50, 50× 75, 50× 100
Set 4
As × Ser = 100× 25), 100×50), 100× 75 and 100ppm× 100ppm.
The soil was air-dried for seven days before being gently hammered to break up large pebbles. The dust was completely mixed after the undesirable components like dried roots, grass, and stones were taken out.
Agronomic parameters
The plant was collected after 21 days, and agronomic aspects like root length and number, shoot length, and leaf numbers were measured and examined.
Investigating the biochemical components
Assessment of biological components
The parameters listed below were measured using a variety of techniques for the biochemical analysis.
Extraction
Fresh leaves were crushed in a mortar with 10ml of distilled water and centrifuged for 10 minutes at 1000 rpm. The supernatant was collected in a new test tube, and the remaining liquid was discarded.
Examining the leaf's protein components
Bradford's Assay reagent was used to extract and measure soluble protein [24].
Extraction of soluble proteins
0.2 g of leaf tissue should be homogenised in 5 ml of phosphate buffer (0.1 m, pH. = 7) using a very cold pestle and mortar. Through cheesecloth or glass wool, filter the homogenised or extracted material. The extract was centrifuged at 4000 rpm for 10 minutes after cooling. The supernatant was transferred to a test tube, and the extract volume was raised to 5 ml using buffer. We diluted the extract by 0.2 mL by adding it to a 4.8 mL buffer (this extract will provide dilution).
Analysis of soluble proteins
There was 0.1 mL of susceptible extract in a check tube. After shaking the extract, 5 mL of Bradford assay were added. With 5 mL of Bradford assay reagent and 0.1 mL of buffer, create a blank reagent. Using a spectrophotometer, the optical density (O.D.) at 595 nm was calculated. The amount of readily soluble protein is determined by an extended curve.
Determination of carotenoids and chlorophyll
Chlorophyll and carotenoids were extracted and quantified using the Maclachlan and Zalik [25] method. A fresh crushed leaf is dissolved in 3 ml of 80% acetone with a tiny quantity of acid-cleared sand, centrifuged for five minutes at 1000 rpm, and then dried. After that, the item is cleaned three times with one milliliter of acetone. Up to 7 mL of the supernatants are mixed with acetone. After that, this data's visual densities at 663nm, 645nm, 480nm, and 510nm were measured.
Equations
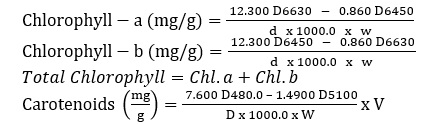
Proline's assessment of Leaves
The leaf proline contents were determined using the Bates et al. [26] method. After being homogenised with % sulphate salicylic acid and filtered through filter paper, 2 ml of extract was combined with 2 ml of glacial acetic acid and 2 ml of ninhydrin acid in a test tube for 1 hour at 100°C. An ice bath was used to end the procedure. After adding 4 ml of toluene and extracting the chromophore for 15 to 20 seconds, the tube was kept in place until all phases had separated. The chromophore that contained toluene was then transferred to a brand-new test tube. At 520 nm, the absorbance was measured. At 520 nm, the absorbance was measured.
Statistical analysis
The one-way ANOVA was performed Least Significant Difference (LSD) at a 5% level using the SPSS 16 (version 16), and graphs were drawn on Origin Pro 10.5v.
Effect of As on roots length of selected crops under the treatment of Ser foliar spray
The effect of As on length of root Abelmoschus esculentus L., Triticum aestivum L., and Zea mays L. under Ser treatments is shown in Table 1. The maximum root length (14.75±1.060) of Triticum aestivum L. was detected at a treatment of 75ppm (As)/50ppm (Ser), while the minimum (10.00±2.82) root length was observed at a treatment of 100 ppm (As)r). In trials conducted in pots, Zea mays L., the greatest root length (17.00±2.82) at treatment was 50 ppm(As)/100 ppm (Ser). Zea mays L. had a minimum (11.60±4.38) root length at treatment of 75ppm (As)/100ppm (Ser). In a similar manner, Abelmoschus esculentus L. data indicated that the maximum length of root was (7.00±0.70) at treatment 75 ppm (As)/100 ppm (Ser), and the minimum root length was 3.00±1.41 at treatment 50 ppm (As). These results suggested that the influence of individual dose of As was maximum, whereas the adverse effect reduced after the foliar spray of Ser.
Effect of As on root number of selected crops under Ser foliar spray
A limited length of time during the vegetative stage was used to evaluate plant samples from the planned experiment under As stress Table 2. After twenty-one days of pot research, the highest root number (14.50±2.12) was discovered, but Triticum aestivum L. had the lowest root number (8.02±0.94) at the same treatment level of 100 ppm (As). According to the Zea mays L. data, the maximum root number was recorded at 14.501±2.02 under the treatment of 100 ppm(As)/100 ppm(Ser), while the lowest root number was 10.503.53 measured under the treatment of 100 ppm(As). Similar to this, the Abelmoschus esculentus L. plant results indicated a maximum root number of 22.00±5.65 at treatment 100 ppm (As)/100 ppm (Ser), and a minimum root number (5.00±1.41) after 21 days at treatment 50 ppm (As) (Table 2). The findings showed that Ser lessened As negative effects.
Influence of As on Shoot Length of selected crops under the Ser treatment
After 21 days of germination, the shoot maximum length (6.00±0.00) of Triticum aestivum L. was detected in treatment at 50 ppm (As)/100 ppm(Ser), while the minimum shoot length was 3.50±0.70 after treatment at 75 ppm (As) (Table 3). In pots investigation for Zea mays L., the maximum shoot length (7.65±1.20) of Zea mays L. was observed in 100 ppm(As)/100 ppm(Ser), whereas the shoot smallest length was 4.25±0.07 at treatment 50ppm (As). Similarly, the maximum shoot length (5.85±0.35) of Abelmoschus esculentus L. was observed at treatment 75ppm (As)/100 ppm (Ser) and the smallest shoot length was 3.65±0.35 at treatment 100ppm (As) (Table 3). These results showed that the Ser reduced adverse effect of As on the shoot length of selected crops.
Effects of As stress on leaf number on selected crops in the field in respect to Serine
Abelmoschus esculentus L., Zea mays L., and Triticum aestivum L. were among the crops assessed for leaf number under As stress in Table 4. For the majority of the treatments, the maximum leaf number of Triticum aestivum L. (5.00±1.00) was noted for 50ppm(As)/100ppm(Ser) and control treatment. The 4.00±0.00 leaf no was absorbed for the remaining treatments. This showed that the influence of As relatively weak on leaf no of Triticum aestivum L. Zea mays L. exhibited its maximum leaf number (5.00±0.00) after 21 days of germination in all treatments with the exception of control, 75, and 100ppm (As). Abelmoschus esculentus L. similarly revealed 3.00±0.00 number of leaves at 21 days for all treatments examined, with the exception of control, 50 and 100 ppm (As). This demonstrated that Zea mays L. and Triticum aestivum L. were less substantially impacted by As than Abelmoschus esculentus L. (Table 4).
As influence on the Chl. “a”, “b” and total Chl. contents (mg/g) of Zea mays L. growing under the Ser applications
The Photosynthetic pigments of Zea mays L. were shown in Figure 1. Chl. "a" contents maximum for the treatments at 50ppm (As)/50ppm (Ser) and at their lowest for the treatments at 100 ppm (As). Similar to this, the maximum Chl. "b" and total Chl. contents were observed for treatments with 50-ppm (As) / 50-ppm (Ser) and minimum for 100-ppm (As). The outcomes demonstrate that Chlorophyll "b" was greater than Chlorophyll "a" in all treatments, whereas Chl. "a," "b," and total Chl. content (mg/g) of Zea mays L. were relatively variable compared to the control treatment. This demonstrated that Ser mitigated the negative effects of As because Chlorophyll "b," in particular, was produced at the highest dosage of Ser.
Effect of As on Chl. "a" and "b" and Total Chl. of Abelmoschus esculentus L. grown in Ser applications
Figure 2 displays the effect of As on photosynthetic pigments of Abelmoschus esculentus L. under the Ser foliar spar. The maximum chlorophyll "a" as observed in treatments of 50ppm (As)/100ppm (Ser), while the lowest was shown with treatments of 25ppm (As). In treatment of 100ppm (As)/100ppm (Ser) had the maximum content of chlorophyll "b" was observed. The maximum total chlorophyll content was reported for 100ppm (As)/100ppm (Ser) treatment and minim total chlorophyll pigment observed at treatment 25ppm(As). These results reveal that Chlorophyll "a" and Chlorophyll "b" responded differently to treatments, but overall Chlorophyll content (mg/g) and Chlorophyll "a" and "b" content (mg/g) of Zea mays L. were increased in various treatments when compared to control treatment.
Effect of As on photosynthetic pigments of Triticum aestivum L. growing under the Ser treatments
Figure 3 shows the influence of As on photosynthetic pigments of Triticum aestivum L. under the Ser foliar spray. The minimal quantity of chlorophyll "a" and “b” observed for the treatment 75 and 100ppm of As, while the greatest content was detected for treatment at 100ppm (As)/100 ppm (Ser). Similarly, the maximum content of total chlorophyll content was observed (Ser) at 100ppm (As)/100 ppm (Ser). According to the findings, higher Chlorophyll "a" than Chlorophyll "b" was formed. This showed that Ser, in particular Chlorophyll "a," prevented As from having a negative impact on Triticum aestivum L. at levels above their limit. These results suggested that Chl “a”, “b”, and Total Chl. contents of Triticum aestivum L. were greater in As+Ser usages as compared to the control and individual doses of As (Figure 3).
Effect of As on protein contents under Ser treatments of selected crops
Results indicated that Zea mays L. treatments at 50-ppm (As) / 100-ppm (Ser) had the highest protein content, followed by treatments at 50-ppm (As) / 50-ppm (Ser) of Abelmoschus esculentus L., and Triticum aestivum L., flowing by treatment 25-ppm (As) / 50-ppm (Ser). The lowest contents of protein were detected for Zea mays L. at treatment 75-ppm (As) / 50-ppm (Ser); Abelmoschus esculentus L., and Triticum aestivum L. at treatment 50-ppm (As), respectively (Figure 4). These results demonstrated that when exposed to produced Ser had a favorable impact on protein content.
Effects of As on proline (g/g) concentration under Ser foliar spray on selected crops
The results indicated that Abelmoschus esculentus L., Zea mays L. and Triticum aestivum L. had the greatest proline contents for treatment, each at 100 ppm (As). The lowest proline concentration Triticum aestivum L. was found in control, which flowed by Zea mays L. at treatment 50-ppm (As) / 100-ppm (Ser) and Abelmoschus esculentus L.at treatment 50-ppm (As)/100 ppm (Ser), respectively (Figure 5). These findings indicate that Ser functions under induced As as an upbeat proline content improver.
Effect of As on carotenoid ( g/g) concentration of selected crops under Ser to Arsenite foliar spray.
The findings revealed that Zea mays L. flowered by Triticum aestivum L. in treatment 75-ppm (As) / 100-ppm had the highest carotenoid content (Ser), followed by Zea mays L. and Triticum aestivum L at treatment 75-ppm (As) / 100-ppm (Ser), respectively (Figure 6). Abelmoschus esculentus L. had the lowest carotenoids content at treatment 50-ppm (As) compared to Zea mays L. and Triticum aestivum L.
Soil analysis for heavy metals
Data suggest that the heavy metals are affected by the addition of Ser and As (Table 5). Higher Mn value observed for treatment As × Ser (100-ppm × 100-ppm) and minimum for control. The higher quantity of Pb and Cr observed for As × Ser (50-ppm × 25-ppm) and As × Ser (100-ppm × 25-ppm), respectively. Similarly, the maximum quantity of Zn was reported for As × Ser (50-ppm × 75-ppm), As × Ser (100-ppm × 25-ppm) for Zn, As (75-ppm) of Ni and Cd. The higher quantity of As was reported for As (100-ppm), whereas low As documented for control treatments (Table 5).
Figures & Tables
In various investigations, the effects of serine treatments on the growth and biochemical makeup of Zea mays L. (maize), Triticum aestivum L. (wheat), and Abelmoschus esculentus L. (okra) under the impacts of arsenic effects have been examined. This research provides insight into serine's capacity to mitigate arsenic's harmful effects on plants. The serine affectedly enhanced growth indices in maize plants under arsenic stress. Compared to plants exposed to arsenic without serine addition, treated plants had longer roots and shoots, greater biomass accumulation, and more chlorophyll [27]. These findings suggest that serine stimulates maize plant development in arsenic-toxic environments. Similar to this, Nahar et al. [28] examined into the effects of serine on arsenic-exposed plant seedlings. In this study, wheat plants treated with serine showed noticeably better growth characteristics, including plant height, leaf area, and wet weight.
These findings suggest that serine treatments can promote wheat plant development under the stress arsenic stress. Similarly, Mairuae et al. [29] investigated the impact of serine on okra plants grown under arsenic-toxic circumstances. This study demonstrated that serine treatment enhanced the growth of the okra plant, including increased plant height, roots, and biomass accumulation. this provide credence to the idea that serine augmentation might encourage okra plant development even in the presence of arsenic-induced stress. Serine treatment has been demonstrated to impact the biochemical constituents of plants subjected to arsenic poisoning in addition to growth metrics. The negative effect of As on photosynthetic pigments depending on the levels of As [5,30,31]. Similarly Rice (Oryza sativa L.) seedlings experience biochemical and molecular changes as a result of chromium stress [32]. Sharma et al. [33] reported that low doses of As (0.25 mg L1) reduced the plant biomass, chlorophyll content, shoot and root elongation relative to control. The serine-treated maize plants had higher amounts of proline and soluble carbohydrates [34]. These substances have significant effects on osmotic control and stress tolerance, suggesting that plants treated with serine have enhanced physiological responses.
Different concentrations of proline applied to the leaves aid in minimising the detrimental effects of different stress [35]. A typical plant response to environmental stresses, such as low light levels and/or heavy metal stress, is proline buildup. Proline metabolism has been proposed to modulate cellular redox status and radical detoxification as additional functions of proline accumulation. [36]. In addition, the study found that plants treated with serine had higher levels of antioxidant enzymes such superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD). A superior defence against oxidative stress brought on by arsenic poisoning is provided by serine supplementation, as evidenced by the increased enzymatic activity [37]. A comparable result was seen in wheat plants that had received serine treatment. In this work, arsenic-induced stress led to higher proline accumulation in wheat plants treated with serine. These findings imply that the antioxidant defence systems may stimulated by serine aid wheat reduce the negative effects of arsenic-induced oxidative stress. The effects of serine on okra plants were studied by Mokgalabone et al. [38]. They discovered elevated proline levels and increased antioxidant enzyme activity, which are comparable to those observed in maize and wheat research. These findings suggest that serine supplementation boosts okra’s ability to withstand stress brought on by arsenic by strengthening its antioxidant defences and osmotic control. There are several pathways that may account for serine’s favourable effects on plant development and the biochemical components of arsenic toxicity. Serine is involved in the production of crucial metabolites including glutathione (GSH) [39], which is crucial for the chelation and subsequent sequestration of arsenic. A precursor for the synthesis of other amino acids including glycine, cysteine, and methionine, which are necessary for the creation of antioxidant enzymes and other protective compounds, serine also plays the role of an amino acid. Although, serine treatments promoted Zea mays L., Triticum aestivum L., and Abelmoschus esculentus L. plant growth and biochemical composition under arsenic poisoning circumstances. The changes in biochemical composition, growth indices, and antioxidant defence systems all point to serine's potential as a stress-relieving compound in the presence of arsenic. However, further research is required to fully understand the underlying processes and to optimise the use of serine to improve crop resistance to arsenic.
A pot experiment was conducted to investigate the effect on plant growth and photosynthetic pigments. The experiment was designed with three replications of thirteen As and As+Ser treatments. Individual (As) treatment of As adversely affect the agronomic parameters of selected planets, whereas the influence of As reduced after the application of Ser. Similarly, the physiological analysis of selected crops such as Triticum aestivum L., Zea mays L. and Abelmoschus esculentus L. showed a minor negative influence after the Ser applications. Photosynthetic pigments, protein, and carotene content of the selected crops were reduced with treatment of As concentrations compared to As+Ser and control treatment. The proline formation was maximum at the higher dosage of As (particularly 100ppm (AS)) in Zea mays L., Triticum aestivum L., and Abelmoschus esculentus L. Low concentrations of heavy metals that are significant for the environment should receive further consideration. As a result, a variety of strategies must be combined with increasingly sensitive detection techniques.
Conflict of Interest
The authors declare no conflict of interest.
References
- Khan MA, Ho Y-S. Arsenic in drinking water: a review on toxicological effects, mechanism of accumulation and remediation. Asian Journal of Chemistry, (2011); 23(5): 1889.
- Toor I, Tahir S. Study of arsenic concentration levels in Pakistani drinking water. Polish Journal of Environmental Studies, (2009); 18(5).
- Kaltreider RC, Davis AM, Lariviere JP, Hamilton JW. Arsenic alters the function of the glucocorticoid receptor as a transcription factor. Environmental health perspectives, (2001); 109(3): 245-251.
- Ali MU, Liu G, Yousaf B, Ullah H, Abbas Q, et al. A systematic review on global pollution status of particulate matter-associated potential toxic elements and health perspectives in urban environment. Environmental geochemistry and health, (2019); 41(3): 1131-1162.
- Zemanová V, Popov M, Pavlíková D, Kotrba P, Hnilička F, et al. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC plant biology, (2020); 20(1): 1-10.
- Tu C, Ma LQ. Effects of arsenate and phosphate on their accumulation by an arsenic-hyperaccumulator Pteris vittata L. Plant and soil, (2003); 249(2): 373-382.
- Zhang W, Cai Y, Tu C, Ma LQ. Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Science of the total Environment, (2002); 300(1-3): 167-177.
- Le XC, Yalcin S, Ma M. Speciation of submicrogram per liter levels of arsenic in water: On-site species separation integrated with sample collection. Environmental science & technology, (2000); 34(11): 2342-2347.
- Qureshi AS. Groundwater governance in Pakistan: From colossal development to neglected management. Water, (2020); 12(11): 3017.
- Nagajyoti PC, Lee KD, Sreekanth T. Heavy metals, occurrence and toxicity for plants: a review. Environmental chemistry letters, (2010); 8(3): 199-216.
- Li C-x, Feng S-l, Yun S, Jiang L-n, Lu X-y, et al. Effects of arsenic on seed germination and physiological activities of wheat seedlings. Journal of Environmental Sciences, (2007); 19(6): 725-732.
- Moulick D, Ghosh D, Santra SC. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiology and Biochemistry, (2016); 109571-578.
- Barrachina AC, Carbonell FB, Beneyto JM. Arsenic uptake, distribution, and accumulation in tomato plants: effect of arsenite on plant growth and yield. Journal of plant nutrition, (1995); 18(6): 1237-1250.
- Islam M, Islam S, Jahiruddin M, Islam M. Effects of irrigation water arsenic in the rice-rice cropping system. J Biol Sci, (2004); 4(4): 542-546.
- Kamran MA, Xu R-K, Li J-Y, Jiang J, Nkoh JN. Effect of different phosphorus sources on soybean growth and arsenic uptake under arsenic stress conditions in an acidic ultisol. Ecotoxicology and Environmental Safety, (2018); 16511-18.
- Abedin M, Meharg AA. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant and soil, (2002); 243(1): 57-66.
- Rahman M. Influence of soil arsenic concentrations on rice (Oryza sativa L.). Subtrop Agric Res Dev, (2004); 2(3): 24-31.
- Chen Y, Han Y-H, Cao Y, Zhu Y-G, Rathinasabapathi B, et al. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Frontiers in plant science, (2017); 8268.
- Jiang QQ, Singh BR. Effect of different forms and sources of arsenic on crop yield and arsenic concentration. Water, Air, and Soil Pollution, (1994); 74(3): 321-343.
- Gunes A, Pilbeam DJ, Inal A. Effect of arsenic–phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant and Soil, (2009); 314(1): 211-220.
- Marin A, Masscheleyn P, Patrick W. The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant and Soil, (1992); 139(2): 175-183.
- Marin A, Pezeshki S, Masschelen P, Choi H. Effect of dimethylarsenic acid (DMAA) on growth, tissue arsenic, and photosynthesis of rice plants. Journal of Plant Nutrition, (1993); 16(5): 865-880.
- Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta, (2002); 58(1): 201-235.
- Hammond JB, Kruger NJ (1988) The bradford method for protein quantitation. New Protein Techniques: Springer. pp. 25-32.
- Maclachlan S, Zalik S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Canadian Journal of Botany, (1963); 41(7): 1053-1062.
- Bates LS, Waldren RP, Teare I. Rapid determination of free proline for water-stress studies. Plant and soil, (1973); 39(1): 205-207.
- Sahay S, Khan E, Praveen A, Panthri M, Mirza Z, et al. Sulphur potentiates selenium to alleviate arsenic-induced stress by modulating oxidative stress, accumulation and thiol-ascorbate metabolism in Brassica juncea L. Environmental Science and Pollution Research, (2020); 2711697-11713.
- Nahar K, Rhaman MS, Parvin K, Bardhan K, Marques DN, et al. Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses, (2022); 2(2): 179-209.
- Mairuae N, Connor JR, Lee SY, Cheepsunthorn P, Tongjaroenbuangam W. The effects of okra (Abelmoschus esculentus Linn.) on the cellular events associated with Alzheimer’s disease in a stably expressed HFE neuroblastoma SH-SY5Y cell line. Neuroscience letters, (2015); 6036-11.
- Kabir A. Biochemical and molecular changes in rice seedlings (Oryza sativa L.) to cope with chromium stress. Plant Biology, (2016); 18(4): 710-719.
- Maglovski M, Gerši Z, Rybanský Ľ, Bardáčová M, Moravčíková J, et al. Effects of nutrition on wheat photosynthetic pigment responses to arsenic stress. Polish Journal of Environmental Studies, (2019); 28(3): 1821-1829.
- Mahdieh S, Ghaderian SM, Karimi N. Effect of arsenic on germination, photosynthesis and growth parameters of two winter wheat varieties in Iran. Journal of plant nutrition, (2013); 36(4): 651-664.
- Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, et al. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules, (2019); 9(7): 285.
- Vasanth K, Lakshmiprabha A, Jayabalan N. Amino acids enhancing plant regeneration from cotyledon and embryonal axis of peanut (Arachis hypogaea L.). Indian Journal of Crop Science, (2006); 1(1and2): 79-83.
- Verslues PE, Sharma S. Proline metabolism and its implications for plant-environment interaction. The Arabidopsis Book/American Society of Plant Biologists, (2010); 8.
- Li X, Li B, Yang Y. Effects of foliar selenite on the nutrient components of turnip (Brassica rapa var. rapa Linn.). Frontiers in Chemistry, (2018); 642.
- Velayutham M, Ojha B, Issac PK, Lite C, Guru A, et al. NV14 from serine O‐acetyltransferase of cyanobacteria influences the antioxidant enzymes in vitro cells, gene expression against H2O2 and other responses in vivo zebrafish larval model. Cell Biology International, (2021); 45(11): 2331-2346.
- Mokgalabone TT, Mpai S, Ndhlala AR. Organic Medium Enclosed Trough Growing Technique Improves Abelmoschus esculentus (Okra) Growth, Yield and Some Nutritional Components. Applied Sciences, (2023); 13(9): 5645.
- Kopriva S, Rennenberg H. Control of sulphate assimilation and glutathione synthesis: interaction with N and C metabolism. Journal of experimental botany, (2004); 55(404): 1831-1842.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0














