Full Length Research Article
Chemo-Modulatory Potential of Flaxseed Oil as Natural Anticancer Therapeutic
Aliza Batool1, Umar Farooq1*, Afshan Shafi1, Zulqurnain Khan2, Rao Muhammad Ikram3, Muhammad Shahbaz1, Nida Firdous1, Mariam Iqbal1, Naqi Abbas1, Zahid Rafiq1
Adv. life sci., vol. 10, no. 3, pp. 362-367, September 2023
*- Corresponding Author: Umar Farooq (Umar.farooq@mnsuam.edu.pk)
Authors' Affiliations
2. Department of Biotechnology, institute of Plant Breeding and Biotechnology (IPBB) MNS-University of Agriculture, Multan – Pakistan
3. Department of Agronomy, MNS-University of Agriculture, Multan – Pakistan
[Date Received: 11/04/2022; Date Revised: 16/03/2023; Date Published: 30/09/2023]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Cancer is a disease which is characterised by uncontrolled cell proliferation and development. Surgery, chemotherapy, radiotherapy, and photodynamic therapy are the most frequent cancer treatments. On the other hand, there are many negative health impacts of radiation and chemotherapy that limit the efficient use of these therapies.
Methods: This scenario needs natural treatments that are cost-effective and has no adverse effects. Flaxseed oil can be used as a nutraceuticals for the management of cancer. The current research was aimed on the exploration of the flaxseed (Linum usitatissimum) oil for in vitro anticancer activity as a natural therapy for the management of cancer.
Result: The flaxseed powder contained 36.6±0.04% oil contents with an average yield of 36.6±0.03% by using hexane as solvent for extraction. Moreover, the oil contained polyunsaturated fatty acid with omega 3 fatty acid (alpha-linolenic acid) as a dominant content of the oil. In-vitro anticancer activity of flaxseed oil was observed by Cytotoxic (3T3 cell line) and Prostate Cancer (PC3 cell line) indicating that the oil possessed anticancer activity which was dose-dependent.
Conclusion: On the basis of results, it was concluded that the flaxseed or its oil can be used for the management of cancer as a natural therapy by using optimized dose levels for a different types of cancer.
Keywords: Flaxseed; Flaxseed oil; Human cancer; Nutraceuticals; Disease management
Cancer is a disease caused by the uncontrolled cell division. The development and progression of cancer cell are basically due to the change in the DNA cell. Under different circumstances, the tumour cell migrates, attacks, and proliferate to other organs and tissue of the body [1]. Overall, worldwide about 19.3 million people diagnose cancer every year and cause 10 million deaths. In developed countries, it is the second disease after cardiovascular with a death rate of 10% globally [1]. Every year, around 3 million new cases of cancer were recorded, in Asia, more than 2 million mortalities due to cancers have been reported. There are different types of cancer and show different symptoms and causes. Some common cancer is breast, kidney, lung cancer, prostate, colorectal, liver, bladder cancer, melanoma and non-Hodgkin lymphoma. Cancer cells start to develop abnormally and increase rapidly to form lumps [2]. Depending on the type of tumour many anticancer treatments are used. The most common treatments used for cancer are surgery, radiotherapy, photodynamic therapy, and chemotherapy. Photodynamic therapy is the combination of photosensitizer agent’s e.g., temoporfin or porfimer sodium with a specific kind of light in an oxygen-rich atmosphere. When photosensitizer agents are exposed to light oxygen is produced and they kill the tumour cell [2]. These treatments show good results but on the other hand, there are many side effects of this therapy. Thromboembolism, nausea, stomatitis, neuropathy, alopecia, myelosuppression, myalgias, and fatigue are the short time effects. And the long-term effect is emesis infertility, weight gain, cardiac dysfunction, cognitive dysfunction and Leukaemia/MDS [3]. A major side effect of these treatments is a failure in ovarian and infertility [4]. Natural treatment is cost-effective, easy to access, highly effective, and has no side effects. Natural compounds extract from plants played significant role in the development of several clinically useful anticancer agents [5]. Herbal drugs or extracts contain many active compounds which interact with other given pharmaceutical drugs to increase the therapeutic effect. Different phytochemicals (quercetin, genistein, curcumin, and catechins) are present in herbal drugs, these phytochemicals increase the therapeutic effect and enhance the bioavailability of the other drug [6,7]. Compounds obtained from a natural source show active potential toward cancer treatment in the past many drugs derived from natural sources uses for the treatment of cancer [8].
Cheese, egg, milk, walnut, fish, and flaxseed are rich sources of omega 3 fatty acids. Omega 3 fatty help to decrease the proliferation of cancer. Flaxseed is one of the highest plant sources of soluble mucilage and α- linolenic acid (omega 3 fatty acid) [9]. Flaxseed contains approximately 36 to 40% of oil content it is become much common nowadays as a functional food due to its many health benefits and help in many diseases coronary heart disease, hormonal conditions, many types of cancer, nerve [10]. Flaxseed oil is a rich source of ALA, which is around 55 to 60% of total fatty acids [11,12].
Keeping in view increasing the incidence of various cancer types all over the world and potential health benefits of flaxseed oil the current study were planned to expect flaxseed oil to cold press and solvent extraction techniques. The extracted oil after analysis was subjected to assess the anticancer potential against prostate cancer.
Compositional analysis of flaxseed and oil extraction and analysis
The percentage composition of flaxseed was determined for crude protein, moisture content, ash, crude fibre, and crude fat according to the method of AOAC [13]. The oil was extracted by two different techniques. For this purpose, the flaxseed was divided in two equal parts. The first portion was used for the oil extraction by solvent extraction method by using n-Hexane whereas the other portion was used for the extraction of oil by the cold press method. In cold press method, the screw presser (Carver Press, USA) was used to extract the oil for 20 min at 40ºC-60ºC and pressure range of 29.4–49.0 MPa. The oil yield was measured by the following equation.
![]()
Saponification and peroxide value
Saponification and peroxide value of flaxseed oil was estimated according to the method of AOCS [14]. The equations used for quantification are as under.
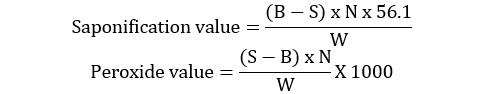
Free Fatty Acids and Refractive index
The free fatty acids and refractive index of flaxseed oil was determined by refractometer as per the recommendations of AOCS [14]. Free fatty acids were calculated by the following formula:
![]()
Fatty acid profile
The fatty acid profile of flaxseed oil was determined by the method of National Institute of Standards and Technology [15]. According to this method, first fatty acid methyl esters were prepared using methanol. With the combination of mass spectroscopy detector (GC-MS) system, 1μL sample of FAME were injected into the gas chromatography (Shimadzu GC-17A). The temperature of the program was set around 70°C to 280°C and helium gas used as a carrier gas. Capillary column (DB-23; 30m x 0.32mm x 0.50μm) performed the separation with flow rate of 0.8mL/min. Firstly, the temperature of the oven was set at 190 °C for the time period of only 1 min then afterwards increased up to 230 °C at a rate of 3 °C min–1. After attaining this temperature, maintained it for 10 minutes. The injection and detector temperatures were kept at 240 and 250 degrees Celsius, respectively. The chromatogram peaks were obtained using retention data from analysed standard samples. Finally, detailed fatty acid content was estimated/calculated as a percentage (%).
Total phenolic contents
The Folin-Ciocalteu assay was used to quantify the total phenol content (TPC) of flaxseed oils [16].
Antioxidant assay
Antioxidant activity of flaxseed oil was determined through spectrophotometer (company, Cecil Instruments limited and model, Aquarius CE 7400S), following method described by Anwar et al. [17]. Antioxidant activity was computed by the given formula:
DPPH radical scavenging (%) = ((AB – AE)/AB) × 100
FTIR Analysis
Infrared spectrum of absorption of flaxseed oil was measured from Fourier-transform infrared spectroscopy (FTIR) techniques model Cary 630 [18].
In-vitro anticancer property of flax seed oil
Cytotoxic (3T3 cell line) and prostate cancer (PC3 cell line) was brought from (HEJ), Husain Ebrahim Jamal Research Institute of Chemistry Karachi. The in-vitro anti-cancer activity of flaxseed oil was determined through the method of Gavamukulya et al., [19].
Statistical Analysis
The data attained during whole study was analysed through analysis of variance (ANOVA) using STATISTIX (Version 8.1) software as recommended by Steel et al. [20], to validate the physio-chemical parameters, anticancer activity of flaxseed oil.
The present research evaluated proximate analysis of flaxseed and then extraction of flaxseed oil was done by two techniques, physio-chemical characterization of flaxseed (Linum usitatissimum) oil and in-vitro anticancer property of flaxseed oil. The results indicated that the flax seed contained 7±0.02%, 18.4±0.04%, 36.6±0.04%, 16±0.06% and 18.2±0.04% of moisture, protein, fat, total minerals and fibre, respectively (Table 1). The oil recovery was 36.6±0.03% and 29.2±0.07% in case of solvent extraction and cold press method, respectively. Similarly, the “L”, “a*” and “b*” values for color of flaxseed oil were relatively higher in the oil extracted through solvent extraction method rather than that of cold press method extracted oil. The “L”, “a*” and “b*” values for solvent extracted oil were 55.2±0.09, 9.10±0.02 and 40.2±0.04 (Table 2). The significantly lowest moisture content was observed in oil extracted by solvent extraction method with mean values of 2.10+0.01% and 0.93±0.01%, correspondingly. The peroxide value was relatively higher in the oil extracted through hexane method rather than that of cold press extracted oil with mean values of 4.13 ± 0.09 mg KOH/g and 3.24± 0.02 mg KOH/g, correspondingly. The highest refractive index value was observed in the oil extracted through cold press method whereas significantly the lowest refractive index value was observed in case of oil extracted through solvent extraction method with mean values of 1.58± 0.01 and 1.17± 0.01. The saponification value of flaxseed oil through hexane was observed to be 181.23± 0.55mg KOH/g whereas saponification value of cold press oil was found to be 177.63± 0.45 mg KOH/g.
The total phenolic contents of flaxseed oil through hexane was observed to be 158±1.16 (mg GAE/g) whereas the total phenolic contents of cold press oil was found to be 90±0.58 (mg GAE/g). The antioxidant activity of flaxseed oil through hexane was observed to be 105±0.58 whereas saponification value of cold press oil was found to be 177.63 ±0.45 (Table 2). The results further exposed that the fatty acid profile of flaxseed oil through hexane was observed to be ALA (50±0.04%), linolenic acid (13±0.02%), oleic acid (18±0.02%) and palmitic acid (3±0.01%) whereas fatty acid profile of cold press oil was found to be ALA (48±0.03%), linolenic acid (11±0.01%), oleic acid (17±0.01%) and palmitic acid (2±0.01%) as shown in table 3.
The results further indicated that the a-linolenic contents were higher than others fatty acids in both oils with mean values of 50±0.04% (SEO) and 48±0.03% (CPO) (Table 3). The oils were also subjected to FTIR analysis and the result obtained are shown in Fig. 1 cold press oil and solvent extraction (hexane) oil as chromatogram.
The peaks above the 3000 cm-1 for alkene and below 3000cm-1 for alkane. The spectra shows that below 1172 cm-1 saturated fatty acid were present, from 1319 cm-1 to 1700 cm-1 monounsaturated fatty acid and from 2841 cm-1 to 3007cm-1 polyunsaturated fatty acid present. Flaxseed oil has been very efficient toward several different kinds of cancer cells. The anti-proliferative effect of flaxseed oil on human prostatic cell lines PC3 was evaluated through MTT assay. MTT assays showed that the flaxseed oil at the concentration of 30 µg/mL for 48 h showed anti-proliferative effect on PC3 cells. The result of oil extracted through cold press were better as compared to hexane extracted. Cytotoxic (3T3 cell line) activity of flaxseed oil was also observed through MTT assay. Oil extracted by both method cold press and hexane extracted were performed Cytotoxic (3T3 cell line) activity. MTT assays showed that the flaxseed oil at the concentration of 30 µg/mL for 48 h cytotoxic effect on 3T3 cell line. The result indicated the cytotoxic activity of flaxseed oil extracted through cold press was higher as compared to hexane extracted. It was also observed the effectiveness of flaxseed oil toward cancer treatment was dose dependent (Table 4).
Figures & Tables
Parallel results were also observed by Aslam et al., [26] during investigation on physicochemical analysis of flaxseed and found the range of moisture contents 5.9-6.1%, crude protein 18.67-20.7%, total ash 6.98-7.02%, crude fiber 38.5-42.55% and crude fat 9.50-11.5%. Another parallel result observed by Ishag et al. [27] were also in well agreement with the current study results. They found the proximate composition of flaxseed as moisture 8.50 ± 0.49%, fat 43.17 ± 0.99%, protein 21.00 ± 0.74%, ash 1.96 ± 0.00%, and fiber 20.23 ± 3.47%. Similar results were also observed by Ishag et al., [27] during investigation on flaxseed oil and found the oil yield of flaxseed from solvent extraction 21.95±0.01% method whereas 18±0.01% yield observe from cold press method. Another parallel result observed by Kulkarni et al., [28] was also in well agreement with the current study results. They found flaxseed oil from solvent extraction 41.53 ± 0.9 method whereas 25.50% ± 0.7 yield observed from cold press method.The overall physiochemical results of current research work are justified by a number of related studies. Comparable outcomes were also observed by Ishag et al. [27] during investigation on physicochemical analysis of flaxseed oil extracted by solvent extraction and found the range of moisture contents0.23+0.01%, refractive index 1.47 ± 0.00,FFA 0.6+0.01%, peroxide value 4.67 ± 1.00 mg KOH/g and saponification value 185.61 ± 0.56 mg KOH/g. While the oil extracted through cold press method contained 0.4570.04 % moisture, FFA 0.6070.08 %, peroxide value 1.24± 0.02 mg KOH/g, refractive index 1.305 ± 0.01 and saponification value 184.21± 0.55mg KOH/g.Another parallel result observed by Shim et al., [29] were also in well agreement with the current study results. They found the proximate composition of flaxseed oil moisture 0.25.04 %, FFA 0.25± 0.01%, peroxide value 0.9± 0.01 mg KOH/g, refractive index 1.295 ± 0.01 and saponification value 190± 0.3mg KOH/g. Grajzer et al., [30], Hernández-Salazar et al., 2013, Herchi et al., [31], Zhang et al., [32], Grajzer et al., [30], Ishag et al., [27], Shim et al. 2015 [29], Khattab and Zietoun [33] and Danish and Nizami [34] also found the similar results in respect of composition and functional properties. Similar observation was also observed by Buckner et al., [24] who also evaluated the anti-cancer effects of flaxseed oil through in vitro study through direct effects on the growth of cancer cell however, it was found to be dose-dependent. The anticancer potential of flaxseed oil might be due to the presence of omega 3 fatty acids in the oil. The results can be justified by the findings of Meng et al., [35], who also observed that the omega 3 fatty acids showed anti-proliferative effect on human prostate cancer. These studies showed that flaxseed oil can specifically inhibit the growth of cancer cell and persuade the process of apoptosis.
In the current study, we explored role of flaxseed’s oil in vitro anticancer activity as a natural therapy for the better management of cancer. The flaxseed powder contained 36.6±0.04% oil contents with an average yield of 36.6±0.03% by using hexane as solvent for extraction. Moreover, the oil contained polyunsaturated fatty acid with omega 3 fatty acid (alpha-linolenic acid) as a dominant content of the oil.In-vitro anticancer activity of flaxseed oil was observed by Cytotoxic (3T3 cell line) and Prostate Cancer (PC3 cell line) indicating that the oil possessed anticancer activity which was dose-dependent. On the basis of results it was concluded that the flaxseed or its oil can be used for the management of cancer as a natural therapy by using on daily basisfor a different types of cancer like breast and prostate.
Acknowledgement
We are thankful to ORIC and Central Lab System, MNS University of Agriculture, Multan (Pakistan) for provision of research facilities and support.
Conflict of Interest
The authors have no conflict of interest
All authors have made a significant contribution to a journal article and share responsibility and accountability for the results. All authors have read and agreed to the published version of the manuscript.
References
- Chhikara BS, Parang K. Global Cancer Statistics 2022: the trends projection analysis. Chemical Biology Letters, (2023); 1: 451-451.
- Maira MS, Pearson MA, Fabbro D, Echeverrıa CG. Cancer Biology In: Comprehensive Medicinal Chemistry II. Elsevier;(2007).
- Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. JNCI Monographs,(2001);30:135-42.
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Human reproduction update, (2001); 6:535-43.
- Zishan M, Saidurrahman S, Anayatullah A, Azeemuddin A, Ahmad Z, Hussain MW. Natural products used as anti-cancer agents. Journal of Drug Delivery and Therapeutics,(2017); 3:11-8.
- HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, (2006); 4:239-49.
- Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, Capaccioli S. Natural compounds for cancer treatment and prevention. Pharmacological research,(2009); 6:365-78.
- Polu P, Nayanabhirama U, Khan S. Herbal medicinal plants as an anticancer agent. Annals of Phytomedicine, (2015); 1:37-45.
- Logarusic M, Radosevic K, Bis A, Panic M, Slivac I, Gaurina Srcek V. Biological potential of flaxseed protein hydrolysates obtained by different proteases. Plant Foods for Human Nutrition,(2020);4:518-24.
- Bozan B, Temelli F. Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresource Technology,(2008);14:6354-9.
- Herchi W, Sakouhi F, Arraez-Román D, Segura-Carretero A, Boukhchina S, Kallel H, Fernandez-Gutierrez A. Changes in the content of phenolic compounds in flaxseed oil during development. Journal of the American Oil Chemists' Society,(2011); 8:1135-42.
- Rubilar M, Gutierrez C, Verdugo M, Shene C, Sineiro J. Flaxseed as a source of functional ingredients. Journal of soil science and plant nutrition,(2010);3:373-7.
- AOAC (2000) Official Methods of Analysis of AOAC International. 17th edition. AOAC International Publishers, USA.
- AOCS (2009) Official methods and recommended practices of the AOCS, 6th edition. American Oil Chemists’ Society, Champaign, IL.
- National Institute of Standards and Technology (NIST). GC-MS NIST Library Information Manual,(2012).
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. Journal of agricultural and food chemistry, (1999);10:3954-62.
- Anwar F, Przybylski R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). ACTA Scientiarum Polonorum Technologia Alimentaria, (2012);3:293-302.
- Rohman A, Man YC. Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food research international, (2010);3:886-92.
- Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pacific journal of tropical medicine, (2014);7:355-63.
- Steel RG. Analysis of variance II: multiway classifications. Principles and procedures of statistics: A biometrical approach,(1997); 2: 204-52.
- Gupta P, Khan MY, Verma VK, Pathak A. Beating Cancer with Natural Plant Sources. Asian journal of Pharmacy and Technology, (2013); 2:39-44.
- Fung TT, Stampfer MJ, Manson JE, Rexrode KM, Willett WC, Hu FB. Prospective study of major dietary patterns and stroke risk in women. Stroke, (2004);9:2014-19
- Key TJ, Schatzkin A, Willett WC, Allen NE, Spencer EA, Travis RC. Diet, nutrition and the prevention of cancer. Public health nutrition,(2004); 1:187-200.
- Buckner AL, Buckner CA, Montaut S, Lafrenie RM. Treatment with flaxseed oil induces apoptosis in cultured malignant cells. Heliyon,(2019); 8:02251.
- Wang L, Chen J, Thompson LU. The inhibitory effect of flaxseed on the growth and metastasisof estrogen receptor negative human breast cancer xenograftsis attributed to both its lignan and oil components. International journal of cancer, (2005); (5):793-8.
- Ayesha A, Khalil AA, Shahid B, Aadil RM, Faiz-ul-Hassan S, Khan AA, Khan MA, Hina G, Ahood K, Quratulain S, Aqsa R. Comparison of proximate composition and mineral profiling of defatted flaxseed cake obtained from three different varieties. Pakistan Journal of Food Sciences,(2019);2:15-8.
- Ishag AO, Khalid AA, Abdi A, Erwa IY, Omer AB, Nour AH. Proximate Composition, Physicochemical Properties and Antioxidant Activity of Flaxseed. Ann Res Review Biol,(2019);1-10.
- Kulkarni NG, Kar JR, Singhal RS. Extraction of flaxseed oil: a comparative study of three-phase partitioning and supercritical carbon dioxide using response surface methodology. Food and Bioprocess Technology,(2017); 5:940-8.
- Shim YY, Gui B, Wang Y, Reaney MJ. Flaxseed (Linum usitatissimum L.) oil processing and selected products. Trends in Food Science and Technology, (2015);2:162-77.
- Grajzer M, Szmalcel K, Kuźmiński Ł, Witkowski M, Kulma A, Prescha A. Characteristics and antioxidant potential of cold-pressed oils—Possible strategies to improve oil stability. Foods,(2020); 11:1630.
- Herchi W, AMMAR KB, Bouali I, Abdallah IB, Guetet A, Boukhchina S. Heating effects on physicochemical characteristics and antioxidant activity of flaxseed hull oil (Linum usitatissimumL). Food Science and Technology,(2016);36:97-102.
- Sun X, Zhang L, Li P, Xu B, Ma F, Zhang Q, Zhang W. Fatty acid profiles-based adulteration detection for flaxseed oil by gas chromatography mass spectrometry. LWT-Food Science and Technology, (2015); 1:430-6.
- Khattab RY, Zeitoun MA. Quality evaluation of flaxseed oil obtained by different extraction techniques. LWT-Food Science and Technology,(2013);1:338-45.
- Danish M, Nizami M. Complete fatty acid analysis data of flaxseed oil using GC-FID method. Data in brief, (2019);23:103845.
- Meng H, Shen Y, Shen J, Zhou F, Shen S, Das UN. Effect of n-3 and n-6 unsaturated fatty acids on prostate cancer (PC-3) and prostate epithelial (RWPE-1) cells in vitro. Lipids in health and disease, (2013);1:1-4.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0








