![]()
Selection of potent bacterial strain for over-production of PHB by using low cost carbon source for eco-friendly bioplastics
Rahat Abdul Rehman1, Abdul Qayyum Rao2, Zahoor Ahmed1, Ambreen Gul2
Adv. life sci., vol. 3, no. 1, pp. 29-35, November 2015
*- Corresponding Author: Rahat Abdul Rehman (Email: rahatrehman@yahoo.com)
Authors' Affiliations
2- Centre of Excellence in Molecular Biology University of the Punjab, Lahore – Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
Supplementary Data
References
Abstract
Background: The microbial PHB production is a promising tool for the plastic industry for the synthesis of environmental friendly, biodegradable plastic in contrast to the conventional petro-chemical based non-degradable plastics. The selection of potent bacterial strains, inexpensive carbon source, efficient fermentation and recovery processes are important aspects that were taken into account during this study.
Methods: Different bacterial strains i.e. Bacillus Spp, P. putida and P. fluorescens were screened for maximum PHB production. Under media optimization, various carbon and nitrogen sources (alone or in combination) were used to achieve the maximum PHB production. Finally the degradation tests of the PHB sheet were also performed to test its biodegradability potential.
Results: Shake flask studies have shown the PHB concentrations upto 7.02, 4.50 and 34.4 mg/g of dry cell mass of P. putida, P. fluorescens and Bacillus Spp. respectively. Almost same results were observed at laboratory scale production of PHB in 10 L fermenter i.e. 6.28, 6.23 and 39.5 mg/g of dry cell mass by P. putida, P. fluorescens and Bacillus Spp. respectively. On the basis of these observations, Bacillus Spp. was chosen for laboratory scale PHB production. Corn steep liquor (4%) was chosen as the best medium to achieve the highest PHB contents. Isolated PHB has shown biodegradation in soil up to 86.7% at 37oC.
Conclusion: The Bacillus Spp. Proved to be the best strain for PHB production on only 4% CSL which is cheapest and easily available.
Keywords: Corn steep liquor, Psuedomonas putida, Polyhydroxybutyrate, Bioplastic, Fermentation, Nile red staining
Introduction
Plastics are xenobiotic agents made of synthetic long chain polymers of polyethylene, polypropylene, polystyrene, poly (vinyl chloride) and poly (ethylene terephthalate) [1]. These polymers are resistant to microbial and enzymatic biodegradation [2]. Being weightless, water resistant and high strength entity, plastics have gradually replaced the cellulose based packaging and wrapping products [2]. Though the plastics have revolutionized the modern era, but being xenobiotics, they are intractable to microbial degradation and pose challenges in their disposal [1]. The very long molecular sizes of plastics create hindrance in their natural biodegradation process in the soil [3]. Through green chemistry evolution, the plastics are engineered to degrade quickly and into environmental friendly byproducts [4]. The petrochemical based plastics resist the natural environmental decay and hence they pollute the environment. However the oil-based, plant-based or microorganism-based plastics comprising the poly-3-hydroxybutyrate (PHB), polyhydroxyvalerate (PHV) and polyhydroxyhexanoate (PHH) undergo the natural degradation process. The D(-)-3-hydroxybutyric acid is the degradation product of PHB and is also a common metabolic intermediate in higher organisms[5]. Polyhydroxyalkanoates are the polyesters of different hydroxyalkanoates. The molecular mass of polyhydroxyalkanoates ranges from 50,000–1,000,000Da.
These are accumulated as storage material in the microorganisms under the state of limiting nutritional elements (such as nitrogen, phosphorous, sulphur, oxygen or magnesium) [6]. PHB, an isotactic polymer, is the well-known member of the PHAs. It exists in a fluid amorphous state within the cell. It is moisture resistant, shows piezoelectric effect and above all is truly and completely biodegradable [7]. The PHB can be cheaply produced using agriculture, dairy or oil industry waste. However, the choice of the strain is an important aspect to take into account while accomplishing the cost-effective production.
In this study the potent bacterial strain was selected for the overproduction of PHB. The low-cost production media was formulated to scale down the production cost of PHB and make it economically feasible.
Methods
Microorganisms and their maintenance
Locally isolated Pseudomonas putida, Pseudomonas fluorescens, and Bacillus spp. were used for screening of PHB. The cultures were propagated and maintained on Luria-Bertani (LB) agar containing (g/100 ml): 1.0 g tryptone, 0.5 g yeast extract, 1.0 g sodium chloride, 1.5 g agar and pH 7.5.
Selection of cheap culture medium for PHB production
Different carbon and nitrogen sources were used for media optimization. The carbon sources included; glucose, sucrose, starch and molasses. The nitrogen source included; yeast extract, ammonium chloride, ammonium sulphate and corn steep liquor. All the media are listed in Table 1. In all cases the pH was adjusted to 7.5. For screening of potential medium for the production of PHB, the experiment was performed with 10% v/v inoculum at 37oC for 72h at 250 rpm in gyratory shaker. Polymer accumulation was monitored by Sudan Black staining.
Effect of inoculum size on PHB production
In the optimized media for PHB production, effect of inoculum size was studied in concentrations of 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9% and 10%. In all the fermentation experiments 6-8 hrs exponential phase culture (observed from growth curve) in LB was used as inoculum. Fermentation was performed at 37ºC and for 48 hrs at 250 rpm on a gyratory shaker after which the culture was harvested, washed, dried, weighed and the PHB was recovered by dispersion method.
Laboratory scale fermentation (PHB production)
The PHB production was carried out in a laboratory scale 10 L stirred tank fermenter in a batch fermentation process. The fermenter contained previously optimized media (4% CSL) and 5% (v/v) inoculum at 37ºC temperature and 250 rpm shaking speed for 48 hrs. Optical density, pH and purity of culture checked at various intervals and the polymer accumulation was monitored by Sudan Black staining. At the end of fermentation, the dry weight of culture was recorded and polymer was recovered.
Cell dry mass
Biomass content was determined by gravimetric method. Culture (10 ml) was centrifuged (6,000 rpm, 10 min, 4oC), cell pellet was washed in deionized water, recovered (6,000 rpm, 10 min, 4oC), dried to constant weight (90oC, 24 h) and weighed.
Nile red staining
Nile red staining was used for the selection of PHB producing bacterial colonies after the method of [8] and proceeded for shake flask studies. Comparatively, high quantities of PHB were produced form Bacillus Spp. so this was the only strain used for further experimentation.
Staining for PHB and spores
Accumulation of PHB granules inside the bacterial cells was detected by staining with Sudan Black B [9]. Approximate estimates of cell contents of PHB were made under oil immersion lens of microscope. The average number and size of the inclusions/cell were recorded in terms of an arbitrary scale of ‘+’ signs. The scale ranged from ‘+’ for an average of a few small inclusions/cell through ‘+’ ‘++’ for increasing number of large inclusions to ‘+++’ for culture in which nearly all the cells were packed with masses of inclusions. Spores were stained with hot malachite green for 5 min, washed and counterstained with safranine for 30 seconds.
Recovery of PHB
PHB was extracted from cells using the method by [10] with a slight modification. Dried cell mass (1 g) was treated with chloroform (50 ml) and 20% sodium hypochlorite (50 ml) at 37oC for 90 min. The dispersion was centrifuged at 5,000g for 20 minutes. Three phases were obtained among which the lowest chloroform phase contained PHB. The chloroform phase was separated by filtration. The chloroform was evaporated, and the polymer was removed and weighed.
Biodegradation test
PHB film was obtained by dissolving the PHB in chloroform at 3% (v/v), spread in an aluminium tray followed by drying in a vacuum oven at 40°C for 24 hrs to obtain a constant weight. Equal quantity of soil was taken in four petri dishes. One was sterilized at 121°C for 20 minutes to be used as control sample.
In order to monitor the effect of temperature the experiment was carried out at 28, 37, and 60°C. At predetermined intervals, polymer specimens were removed from the soil, cleansed, and dried in a vacuum oven. Dried films were weighed to calculate the weight loss.starting the experiment. Saline (0.9% NaCl solution; 1 ml/kg) was injected to control animals.
Results
Screening and isolation of PHB
Screening of PHB producing strain:
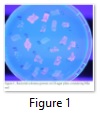
Best PHB producing strain was selected by fluorescence on Nile Red plates (Figure 1) and on the basis of PHB recovery. Bacillus Spp. was found best for PHB accumulation. It accumulated 5-7 times more PHB content as compared to P. putida and P. fluorescens.
Selection of cheap media for PHB production
Various combinations of carbon and nitrogen sources were screened for PHB production and sporulation (Table 1). Yeast extract resulted in significant increase in biomass as was observed microscopically but there was little accumulation of polymer. In glucose containing media, there was no PHB production. However, the addition of sucrose proved to be a stimulant for PHB production with a concomitant increase in the cell biomass. All the experiments with corn steep liquor (CSL), supplemented with different carbon sources showed polymer accumulation to various extents. However, cell biomass increase was observed only in combinations with yeast extract. As can be seen in Table III, increasing the concentration of yeast extract from 0.50 to 1% while keeping the concentration of CSL constant resulted in a decrease in polymer accumulation from 3 to 4 granules per cell to 1 or none at all. Maximum accumulation was observed in medium-14 (CSL4%) just after 24h due to start of sporulation. Commencement of sporulation is an indication of nutrient exhaustion. Therefore, in the next experiment the concentration of CSL was increased to 5% (medium-15). Interestingly, not only the polymer accumulation was decreased, the cell also started lysing thus releasing granules and spores.
Effect of inoculum size
Following the results of shake flask experiments, medium 14 and 15 (Table 2) were selected for further studies. Fermentations were carried out in 2 L flasks for 48 hrs with different inoculum sizes (1-10%). Effect of inoculum size on the PHB production in the two cultural conditions is depicted in figure 2(a) and 2(b). The comparison of both media led to the selection of 5% inoculum as best for PHB production. In contrast to microscopic observations, the medium containing 5% CSL and yeast extract did not give any significant amount of PHB with any of the inoculum size when recovered by dispersion method although the biomass increase was there. Whereas, in case of medium containing 4% CSL resulted in increased polymer content though biomass increase was not so significant.
Comparison of PHB accumulation by different strains
Different bacterial strains were used for comparison of PHB accumulation in optimized medium (4%CSL) at 37oC and 200 rpm for 48 hrs. At the end of fermentation total biomass was 3.44 g/L, 4.98 and 6.70 g/l in Bacillus spp, P. putida and P. fluorescens respectively while the PHB content was 34.4 mg/g, 7.02 and 4.50 mg/g by Bacillus spp, P. putida and P. fluorescens respectively. It is clear that Bacillus spp showed highest PHB accumulation under optimized fermentation conditions (Table 3).
Lab-Scale production of PHB
After the optimization of fermentation conditions in shake flask, the final fermentation was carried out in 10 L stirred-tank fermenter at 37oC and 200 rpm for 48 hrs with medium containing 4% CSL (Table 4). At the end of fermentation total biomass and PHB were 4.36 g/L and 39.5 mg/g, respectively. The pH dropped from 7 to 5.5 after 4 h of inoculation. OD600 was 0.233 after 4 h of inoculation and increased to 2.51 at the time when the culture was harvested. Sudan black staining showed that the cells were loaded with granules at 24 h in CSL 4% as compared to culture grown in LB (Figure 3). It is clearly visible in the micrographs that by the time the sporulation started in the fermentation medium (24 hrs) the culture in LB had little accumulation of PHB. Lysis of cells and the spores in the surroundings is prominent in 48 hrs culture in 4% CSL medium.
Biodegradation test
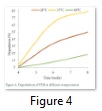
Biodegradation of PHB was characterized by the soil burial experiment in the lab. Figure 5 shows the weight loss of polymers buried in soil for 4-8 weeks 8.5- 57.3% at 28oC, 11.6- 86.7% at 37oC and then decreased at 60oC 7.2% – 25.9%. PHB degradation in soil after 8 week is shown in figure 5(a), 5(b) and 5(c) at 28, 37 and 60oC respectively.
Data and Tables
Discussion
Nutritional and environmental stresses can result in metabolic fluctuations in bacteria. Bacteria respond to these stresses by synthesizing intracellular or extracellular metabolites. During growth-limiting conditions bacteria synthesize and accumulate intracellular polyesters as storage material called polyhydroxyalkanoates (PHAs) [11]. The best polyester among these PHAs is polyhydroxybutyrate (PHB) because of several reasons like its similarity to synthetic based plastics such as polypropylene [12]. Currently, the gram negative bacteria such as Cupriavidus necator [13] Alcaligenes latus and recombinant Escherichia coli [14] are being used for PHB production. But it is observed that PHB produced by Gam negative bacteria contain lipopolysaccharides (LPS) endotoxins as outer membrane which is involved in induction of strong immunogenic reaction and is undesirable for its use in biomedical science [15]. Due to absence of lipopolysaccharides layer in Gram positive bacteria (Bacillus), it is advantageous for use in biomedical applications as it can grow at cheaper substrates and can produce larger quantities of proteins [16-18].
In present study, same attempt was opted to screen optimal PHB producing strain. From the results it is clearly evident that Bacillus Spp. was best for PHB accumulation. Bacillus spp. yielded 34.4 mg/g of PHA as compared to 4.50 mg/g by P. fluorescens and 7.02 mg/g by P. putida under optimal fermentation conditions. The results of this study are consistent with various other studies as reported by [11,16 ,19 ,20] in which they reported a number of Bacillus species to accumulate 9 to 67% (Cell Dry Weight) CDW of PHA.
Reduction of overall cost was the second goal of this study. Different strategies are being adopted by scientist for cost reduction like use of inexpensive renewable carbon substrates for high productivity [21]. In the present study an effort was done to produce PHA at low cost using corn steep liquor as growth medium. Out of various combinations of C/N sources used in the study, 4% CSL alone proved to be the best raw material for maximum production of PHB. These results coincide with results presented by [12,6] in which they obtained high yield of PHA by using low cost agro-industrial by-product and sea water. PHB production in 10 L stirred-tank fermenter under optimum conditions in 4% CSL medium also indicate that total biomass and PHB were 4.36 g/L and 39.5 mg/g, respectively. Medium optimization by application of statistical optimization, compared to the common “one-factor-at-a-time” method, proved to be effective tool [2,6]. Biodegradation of PHB under soil was checked at different temperatures and observed its highest degradation (11.6 – 86.7%) at 37°C within 4-8 weeks which is consistent with the findings by Kim et al [27]. The biodegradable property of PHB is gaining much attention of researchers as an alternate to conventional plastics. The potential applications of PHB in various industries and in the medical field are encouraging. However the production cost of PHB is a major drawback. In the current study, an immense progress has been made in searching for new bacterial strains, creating new types of recombinant strains and by utilization of cheap carbon and nitrogen sources to reduce the cost of production. The ongoing commercialization activities in several countries are expected to make PHB available for applications in various areas.
Acknowledgement
I gratefully acknowledge the financial support from Higher Education Commission of Pakistan for the commencement of this study at the institute of School of Biological Sciences, Punjab University Lahore.
References
- Flechter A In: Plastics from Bacteria and for Bacteria: PHA as Natural, Biodegradable Polyesters. (1993); p 77-93. Springer Verlag, New York.
- Raaz M, Bina R, Parihar S, Sharma A. Eco-friendly Bioplastic for Uncontaminated Environment. Research Journal of Chemical and Environmental Sciences, (2013); 1(1): 44-49.
- Ronald M. Atlas RB Microbial Ecology: Fundamentals and Applications. (1993)
- Barron K. Green Chemistry and Biodegradeable Plastics. ESSAI, (2011); 8(1): 7.
- Lee S Y. 1996. Plastic bacteria? Progress and prospects for poly-hydroxyalkanoate production in bacteria. Trends. Biotechnol.14:431-438..
- Disha N, Bhawana P, Fulekar MH. Production of Biodegradable Plastic from Waste Using Microbial Technology. International Journal of Research in Chemistry and Environment, (2012); 2(2): 118-123.
- Lauzier C, Revol J F and Marchessault, R H. Topotactic crystalization of isolated poly -3- hydroxybutyrate granules from Alcaligenes eutrophus. FEMS Microbiol. Rev, (1992); 103:299- 310.
- Tyo, K E, Hang Z, and Gregory N. S. "High-throughput screen for poly-3-hydroxybutyrate in Escherichia coli and Synechocystis sp. strain PCC6803." Applied and environmental microbiology 72.5 (2006): 3412-3417.
- Kumari P, Dhingra HK. Isolation and characterization of PHB producing micro-organisms isolated from root nodules of leguminous plants. The Bioscan, (2013); 8(1): 109-113.
- Hahn SK, Chang YK, Kim BS, Chang HN. Optimization of microbial poly (3‐hydroxybutyrate) recover using dispersions of sodium hypochlorite solution and chloroform. Biotechnology and Bioengineering, (1994); 44(2): 256-261.
- Reddy SV, Thirumala M, Mahmood S. Production of PHB and P (3HB-co-3HV) biopolymers by Bacillus megaterium strain OU303A isolated from municipal sewage sludge. World Journal of Microbiology and Biotechnology, (2009); 25(3): 391-397.
- Mokhtari-Hosseini ZB, Vasheghani-Farahani E, Heidarzadeh-Vazifekhoran A, Shojaosadati SA, Karimzadeh R, et al. Statistical media optimization for growth and PHB production from methanol by a methylotrophic bacterium. Bioresource Technology, (2009); 100(8): 2436-2443.
- Vandamme P, Coenye T. Taxonomy of the genus Cupriavidus: a tale of lost and found. International Journal of Systematic and Evolutionary Microbiology, (2004); 54(6): 2285-2289.
- Choi J-i, Lee SY, Han K. Cloning of the Alcaligenes latus polyhydroxyalkanoate biosynthesis genes and use of these genes for enhanced production of poly (3-hydroxybutyrate) in Escherichia coli. Applied and Environmental Microbiology, (1998); 64(12): 4897-4903.
- Chen G-Q, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials, (2005); 26(33): 6565-6578.
- Valappil SP, Misra SK, Boccaccini AR, Keshavarz T, Bucke C, et al. Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. Journal of Biotechnology, (2007); 132(3): 251-258.
- Lopes MSG, Rocha RCS, Zanotto SP, Gomez JGC, da Silva LF. Screening of bacteria to produce polyhydroxyalkanoates from xylose. World Journal of Microbiology and Biotechnology, (2009); 25(10): 1751-1756.
- Singh M, Patel SK, Kalia VC. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microbial Cell Factories, (2009); 8(1): 38.
- Łabużek S, Radecka I. Biosynthesis of PHB tercopolymer by Bacillus cereus UW85. Journal of Applied Microbiology, (2001); 90(3): 353-357.
- Tajima K, Igari T, Nishimura D, Nakamura M, Satoh Y, et al. Isolation and characterization of Bacillus sp. INT005 accumulating polyhydroxyalkanoate (PHA) from gas field soil. Journal of Bioscience and Bioengineering, (2003); 95(1): 77-81.
- Ojumu T, Yu J, Solomon B. Production of polyhydroxyalkanoates, a bacterial biodegradable polymers. African Journal of Biotechnology, (2004); 3(1): 18-24.
- Kim P, Kim J-H, Oh D-K. Improvement in cell yield of Methylobacterium sp. by reducing the inhibition of medium components for poly-β-hydroxybutyrate production. World Journal of Microbiology and Biotechnology, (2003); 19(4): 357-361.
- Ishizaki A, Tanaka K, Taga N. Microbial production of poly-D-3-hydroxybutyrate from CO2. Applied Microbiology Biotechnology, (2001); 57(1-2): 6-12.
- Nikel PI, Pettinari MJ, Méndez BS, Galvagno MA. Statistical optimization of a culture medium for biomass and poly (3-hydroxybutyrate) production by a recombinant Escherichia coli strain using agroindustrial byproducts. International Microbiology, (2010); 8(4): 243-250.
- Nath A, Dixit M, Bandiya A, Chavda S, Desai A. Enhanced PHB production and scale up studies using cheese whey in fed batch culture of Methylobacterium sp. ZP24. Bioresource Technology, (2008); 99(13): 5749-5755.
- RamKumar Pandian S, Deepak V, Kalishwaralal K, Rameshkumar N, Jeyaraj M, et al. Optimization and fed-batch production of PHB utilizing dairy waste and sea water as nutrient sources by Bacillus megaterium SRKP-3. Bioresource Technology, (2010); 101(2): 705-711.
- Kim M-N, Lee A-R, Yoon J-S, Chin I-J. Biodegradation of poly (3-hydroxybutyrate), Sky-Green® and Mater-Bi® by fungi isolated from soils. European Polymer Journal, (2000); 36(8): 1677-1685.