Full Length Research Article
The role of Vitamin C and Ferrlecit in Reducing of Micronucleus induced by MTX Chemotherapy
Alyaa Abdulhadi Salih*, Lina Mussa Kadhim
Adv. life sci., vol. 11, no. 1, pp. 119-124, February 2024
*- Corresponding Author: Alyaa Abdulhadi Salih (alyaasaleh@uowasit.edu.iq)
Authors' Affiliations
[Date Received: 02/08/2023; Date Revised: 24/12/2023; Date Published: 25/02/2024]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Methotrexate (MTX) has been used for a long time as rheumatoid arthritis medication as well as for the treatment of several malignancies, including hepatoma, lymphoma, treatments of MTX and other anticancer medications are now being considered and used for various tumor treatments in order to lessen MTX-induced adverse effects and boost anticancer efficacy. As a result, the current work suggests a potentially strong therapeutic strategy for malignancies involving the use of vitamin C, ferret (Sodium Ferric Gluconate), and MTX.
Methods: Totally, 30 Albino Swiss male mice were selected and divided equally into 6 groups; negative control (no drug administered), positive control (received a single 20 mg/kg dosage of MTX, in addition to four treated groups; group 1: Vitamin C 50 mg/kg, group 2: ferrlecit 30 mg/kg, group 3: Vitamin C + MTX and group 4: ferrlecit + MTX. Samples were collected 48 hr. following the last dose of the medication. Smears were collected and Giemsa-stained. To analyze micronuclei, 1000 polychromatic erythrocytes (PECs) were counted in each animal.
Results: MTX increased cytotoxicity in the bone marrow, which was shown by a marked increase in MN formation in contrast to control. Statistically substantial decreases in percentage of micronuclei were seen in both combination pretreatment groups (MTX and Vit C) and (MTX and Fe) in comparison to mice given MTX alone. Ferrlecit was less efficient than vitamin C at preventing MTX-induced micronuclei.
Conclusion: This study concluded that the combination pretreatment has a considerable anti-toxicity impact on genetic damage. This therapeutic approach may be beneficial for future clinical cancer therapy.
Keywords: Polychromatic erythrocyte; Sodium ferric gluconate; Cytotoxicity; Bone marrow
Although chemotherapy is one of the most efficient cancer treatment options, it is frequently associated with several toxicities both in the short and long term [1]. Methotrexate (MTX) is a chemotherapy drug that is used to treat acute lymphoblastic leukemia (ALL), osteosarcoma, breast cancer, lymphoma, neck and head malignancies, as well as non-oncologic conditions such as rheumatoid arthritis and psoriasis [2]. Methotrexate’s therapeutic efficiency is based on the dihydrofolate reductase (DHER) inhibition, a critical enzyme in the metabolism of folic acid (FA) that converts dihydrofolate to tetrahydrofolate [3]. A disruption in the metabolism of folic acid results in the depletion of nucleotide precursors such thymidylate and purine and this in turn prevents the synthesis of DNA, RNA, and protein. Additionally, methotrexate blocks thymidylate synthase and the entry of decreased folatase into cells [4, 5]. MTX has been demonstrated in several drugs toxicity tests to cause genetic damage, like chromosome abnormality, gene mutation, and DNA damage [6,7].
MTX has also been connected to malignant cells developing DNA damage, including oxidative damage in colon cancer and double-strand breaks (DSBs) in non-small lung cells carcinoma. [8,9]. The micronucleus assay is a quick in vivo and in vitro screening cytogenetic examination that is commonly used to determine the genotoxicity of substances in organisms. [10]. Micronuclei (MN) form when an acentric fragment or entire chromosome fails to integrate into the daughter nuclei in mitosis. In order to quantify clastogenic or aneugenic chromosome DNA injury, MN in the cytoplasm of the interphase daughter cell has been counted [11, 12]. There is substantial evidence that several dietary elements can modify the effect of mutagenic or carcinogenic substances. Vitamin C (Vit. C) is an important dietary component that serves as a cofactor for numerous enzymes as well as a powerful antioxidant that scavenges reactive oxygen species (ROS) and protects cells from free radical damage [13]. In addition to having an antioxidant effect, VC can increase the elimination of damage caused by oxidation from the DNA and/or nucleotide pool by upregulating repair enzymes [14]. Several researchers have demonstrated the inhibitory effects of VC on a variety of mutagens and carcinogens in human as well as animals [15-17]. Ferrlecit (sodium ferric gluconate) is an injectable iron medication used to treat anemia caused by iron deficiency. It is commonly used in hemodialysis patients, erythropoietin treatment patients; along with those suffering from chronic renal disease [18]. Iron is the most prevalent transition metal in our bodies and an essential micronutrient that is required by many important enzymes. Consequently, it is crucial to numerous biological activities, including the synthesis and repair of DNA, oxygen transport, regulating the cell cycle, and energy production [19].
The current study aims to evaluate the protecting effects of Vit C and ferrlecit against the cytotoxicity induced by methotrexate in mice bone marrow.
Ethical approval
The study was approved by the Scientific Committee of the Department of Biology in the College of Science (University of Wasit, Wasit, Iraq)
Study animals
A total of 50 male albino Swiss mice were selected, acclimated and subjected to the present study that conducted during October (2021) to March (2022).
Chemicals
Methotrexate (Ebewe, Austria), Vitamin C, and Ferrlecit® (sodium ferric gluconate) were supplied from Al-Karamah Hospital. To prepare the needed dose for mouse injection, all chemicals diluting in distilled water and administered intraperitoneally. The calculated dose was dissolved in 1 ml of distilled water.
Experimental animals
A total of 30 albino Swiss male mice were gained from the Ministry of Health -Baghdad, the National Center for Drug Control and Research. They were between 10-15 weeks old and 30±2 grams in body weight. The animal understudy was classified into six groups, and each group was housed in its own plastic cage in the biology department's animal house under ideal conditions.
Study Protocol: Six groupings were created out (5 animals for each group) and administered as intraperitoneally single daily doses as follows:
Control group
Group I: Negative control, mouse was received the same amount of distilled water.
Group II: positive control, 20 mg/kg B.W. dose of MTX as single intraperitoneal (i.p.) dose and sacrificed after 48hrs.
Treatment groups
- Group 1: Vitamin C was given to a mouse in dose 50 mg/kg for 5successive days
- Group 2: Ferrlecit was given to a mouse in a dose 30 mg/kg for 5successive days
- Group 3: Mouse pretreated with vitamin C 50 mg/kg for 5 successive days) then administrated with methotrexate 20 mg/kg, i.p. single dose on the sixth day of the experiment.
- Group 5: Mouse pretreated with ferrlecit 30 mg/kg for 5 successive days then administrated i.p. with MTX 20 mg/kg, as a single dose.
Forty-eight hours after the MTX injection, the mouse was sacrificed, and the samples of bone marrow were collected to be taken for cytogenetic analysis (MN).
Micronucleus (MN) Analysis
The following steps were taken to conduct this experiment using Schmid's technique [20]:-
After the mouse is sacrificed, all tissues and muscles are removed from the femur bone. The bone marrow was washed and dropped into the test tube by injecting 1 ml of heat-inactivated human plasma into the femur bone. The tube centrifugate for 5 minutes at (1000) rpm. A pellet drop was employed to make a smear on a clean slide, after the supernatant had been removed. The slides were kept overnight at room temperature; and then, fixed for 5 min with absolute methanol. Giemsa stain was applied to one side for (15 minutes), followed by a D.W. rinse and drying time. For each mouse, two slides have been prepared, and then investigated under the oil immersion lens. For the micronucleus test, at least (1000) polychromatic erythrocytes (PCE) were checked for the presence of micronuclei. The following equation was used to calculate the micronucleus index:
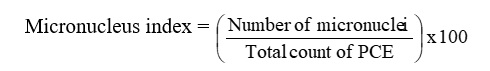
Statistical analysis
Data were translated into a computerized structure. The data is presented as mean ± standard error, and normally distributed an unpaired two-sample T-test was used to compare between two values. SPSS version 25 and Microsoft Excel 2010 computer software were used for conducting statistical analysis. When the probability value was below 0.05, the difference was regarded as significant [21].
Cytotoxicity was assessed by measuring the percentage of total polychromatic erythrocyte (PCE) to total normochromatic erythrocyte PCE / NCE of the control and all the treated groups. In the current study, 1000 PCEs and corresponding normochromic erythrocytes (NCEs) were assessed for each animal ,5 mice to each group = 5000 PCEs to demonstrate the effect of MTX drug at 48 hr after treatment and combination treatment used Vit. C and Fe. A Micronucleus study was conducted based on the rate of incidence of micronucleated MNPCEs out of the total 1000 normal PCEs for each animal. MNPCEs had a small nucleus dark-blue in color as inherited residual material that remains after the erythropoiesis process, two types of MN extracellular and intracellular depended on the site of MN (Figs. 1-a,b).
The administration of 20mg/kg i.p. MTX to mice caused cytotoxicity in the bone marrow, as demonstrated by a significant increase in MN formation (6.470 + 0.32) compared to the control group (1.520+ 0.22) in a p-value less than 0.05. The result of MN for the Vit. C treated group was significantly different versus with the group of negative control, whereas the MN value remained unchanged in the Fe group in contrast to a negative control (Table 1).
On the other hand, the results showed a high significant difference in MN percentage induced by MTX ( 6.470 + 0.32) when compared with the other two treated groups Vit C and Fe (0. 826+ 3.20 and 1.252+ 1.40 ) respectively (Figure 2).
Effects of Combination Treatment on MNs
The mice were pretreated with Vit. C 50 mg/kg/day and Fe 30 mg/kg/day for 5 days before receiving MTX 20 mg/kg/day as a single dose on the sixth day. This treatment helped reduce the adverse effects of MTX in the bone marrow. This improvement was revealed by a significant decrease in MN formation. (3.84+ 2.30 and 4.72+ 6.20%) respectively compared with MTX group (6.470 + 0.32). This comparison illustrates the dose-related impact of Vit. C and Fe against the damage of MTX chemotropics (Table 2). However, the MTX and vit. C as well as the MTX and ferrlecit groups showed a significant decrease in the micronuclei numbers in comparison with mice administrated with MTX alone. Also, the results showed that vitamin C was more efficient than ferrlecit in reducing cytotoxicity induced by MTX.
Figures & Tables
The micronucleus assay (MN), which has been in use for about 40 years, is one of the most widely used techniques for determining the genotoxicity of various chemical and physical variables, including DNA damage brought on by ionizing radiation. [22]. MN was observable
in the cytoplasm of cells as a tiny, chromatin-containing spherical body [23]. DNA damage or genetic instability is the primary causes of MN formation [24]. Additionally, it may emerge from physiological processes like metabolism or aging or may be brought on by several environmental variables, risky behaviors, and various diseases. Therefore, MN was found in little amounts in the control group which was treated with distilled water only.
The findings of this investigation demonstrated a highly substantial rise in the mean of MNPCEs in animals administrated with MTX versus the control. Significant genetic harm caused by MTX was demonstrated by an increase in chromosomal aberration and micronuclei production in both people and animal models [25]. Micronuclei frequencies significantly increase in patients of lymphoblastic leukemia after being treated with anti-leukemia agents like methotrexate and vincristine, this result was recorded by Acar and his colleagues [26].
Other previous results confirm our study, Maleek, and Gheni [27], reported a statistical rise in MN formation in mice after being injected with MTX according to their explanation, the deoxyribonucleoside triphosphate (dNTP) synthesis was inhibited, which led to the development of genetic lesions as a result of a deficiency in DNA repair. However, as the dNTP’s synthesis failed, the genetic damages generated by MTX are manifested as MN. We observed nearly the same effect of MTX in vivo on the frequency of MN in peripheral blood cells as that seen by Novakovic et al. [28]. Nevertheless, methotrexate can also affect the body’s healthy cells and organs, a side effect known as methotrexate toxicity, the risk of toxicity increases with prolonged use of methotrexate and the length of time it remains in the body [29].
MN, which was around four times the control value was induced by the MTX medication alone. No cytotoxic effect was seen with any of the Fe or vitamin C dosages. In combination with MTX, the administration of vit C and Fe both dramatically decreased the impact of MTX by lowering MN counts. This meliorative effect of Vit. C may be due to the improvement of detoxification pathways that transform this reactive chemical into less toxic and more easily eliminated compounds, or it may be due to its effectiveness as a free radical scavenger.
Previous research showed the antioxidant effect of Vit. C either scavenges free radicals or functions as a precursor to oxidation, generating hydrogen peroxide and free radicals [30]. Ascorbic acid, as a physiological substance, aids in the natural antioxidant protection of cells. Additionally, ascorbic acid is regarded as the most significant plasma antioxidant. [31]. According to numerous studies, Vit C has a protective effect against substances that cause cytotoxicity, including medications and chemical agents. [32, 33]. Therefore, Vit C has the potential to neutralize ROS or reactive nitrogen species created by MTX, reducing their availability to interact with intracellular organelles, and genetic material DNA and RNA by preventing the of lipid peroxidation [24].
These results are in compliance with previous observations in mixing Vit. C with other chemotherapy [34]. The current findings are comparable to those of Giri [34]. The current findings are comparable to those of Giri [35], who observed that ascorbic acid protected against chromosomal abnormalities and micronuclei caused by cisplatin in mouse bone marrow cells. In a different study, Aly and Doniya (2002) examined the effects of rifampicin (RMP) on the production of chromosomal abnormalities and discovered that the incidence of these aberrations was dramatically reduced in mice pretreated vitamin C and RMP.
On the other hand, pretreatment with ferrlecit (Fe) and chemotherapy considerably reduced the impact of MTX, but not as significantly as Vit. C [36]. However, ferrlecit (iron), a significant trace element, is essential for energy metabolism, muscular function, hematopoiesis, oxygen metabolism and uptake, and electron transport in mitochondria [37]. Additionally, all eukaryotes require iron as a micronutrient because it functions as a redox cofactor in crucial biological processes. It is now identified as a necessary component of several enzymes involved in basic DNA metabolism processes [38]. DNA helicases, nucleases, glycosylases, demethylases, replicative DNA polymerases, primase, ribonucleotide reductases, and glycosylases are some of the enzymes necessary for DNA synthesis and repair that contain functionally significant iron cofactors. Genetic changes that result in iron cofactor deficiencies can be fatal or cause DNA damage, cancer, developmental, and aging-related illnesses [39]. Therefore, the combination pretreatment for Fe and MTX reduced the aberration in the bone marrow which is represented by MN. This may be due to the protective effect of Fe on the DNA from MTX toxicity.
The cytotoxic effect of MTX in mice bone marrow is mediated by the formation of MN. By exerting antioxidant properties, the combination of Vit. C, Fe with MTX lowers the cytotoxicity caused by MTX. Compared to pretreatment with Fe, pretreatment with Vit. C before administering MTX had stronger synergistic protective effects. In this context, preclinical and clinical investigations are required to prove the combination protective effect of vitamin C and Fe against cytotoxicity induced by MTX.
Conflict of Interest
The authors declare that there is no conflict of interest.
The authors confirm their contribution to the paper as follows:
Alyaa Abdulhadi Salih: Study conception and design; performed analysis and interpretation of results.
Lina Mussa Kadhim: Data collection; draft manuscript preparation .
Both authors wrote the paper, reviewed the results and approved the final version of the manuscript
- Arnon J, Meirow D, Roness HL, Ornoy R. A Genetic and teratogenic effects of cancer treatments on gametes and embryos. Human Reproduction Update, (2001); 7(4): 394–403.
- Mikkelsen TS, Thorn CF, Yang JJ, Ulrich CM, French D, Zaza G, Dunnenberger HM, Marsh S, McLeod HL, Giacomini K,Becker ML, Pharm GK. Summary: methotrexate pathway. Pharmacogenetics and genomics, (2011); 21(10): 679-689.
- Wong PT, Choi SK. Mechanisms and implications of dual-acting methotrexate in folatetargeted nanotherapeutic delivery. International Journal Molecular Sciences, (2015); 16(1): 1772-1790.
- Novakovic T, Dordevic OM, Grujicic D, Marinkovic D, Jankovic S , Arsenijevic S .Effect of intratumoral application of methotrexate in vivo on frequency of micronuclei in peripheral blood lymphocytes. Archive of Oncology, (2003); 11(1):1– 4.
- Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bulletinof the NYU Hospital for Joint Disease, (2007); 65(3): 168-173.
- Deng H, Zhang M, Hetl J. Investigating genetic damage in workers occupationally exposed to methotrexate using three genetic end-points. Mutagenesis. (2005); 20(5): 351–357.
- Shahin AA, Ismail MM, Saleh AM, Moustafa HA, Aboul-Ella AA, Gabr HM .Protective effect of folinic acid on low-dose methotrexate genotoxicity. Zeitschrift fur Rheumatologie, (2001); 60(2): 63–68.
- Martin SA, McCarthy A, Barber LJ. Methotrexate induces oxidative DNA damage and is selectively lethal to tumour cells with defects in the DNA mismatch repair gene MSH2. EMBO Molecular Medicine, (2009); 1(6–7): 323–337.
- Huang WY, Yang PM, Chang YF, Marquez VE, Chen CC. Methotrexate induces apoptosis through p53/p21-dependent pathway and increases E-cadherin expression through downregulation of HDAC/EZH2. Biochemical Pharmacology, (2011); 81(4): 510–517
- Aditya M, Vishhal G, Hemant N, Vishal Sh. Protective Effect of (Curry Leaf) leaves extract against genotoxicity induced by cyclophosphamide in mouse bone marrow cells. Global Veterinaria,(2013); 1(10): 128-133.
- Schmidt W. The micronucleus test. Mutation Research, (1975); 1(31): 9–15.
- Heddle JA. A rapid in vivo test for chromosomal damage. Mutation Research, (1993); 1(18): 187–190.
- Sanchez-Moreno C, Paniague M, Madrid A, Martin A. Protective effect of vitamin C against the ethanol mediated toxic effects on human brain glial cells.The Journal of Nutritional Biochemistry, (2003); 1(14): 606-613.
- Cooke MS, Evans MD, Podmore ID, Podmore KE, Herbert KE, Mistry N, Mistry P, Hickenbotham PT, Husseini A, Griffiths HR, Lunec J . Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Letters, (1998); 363(4): 363¬367.
- Mooney LA, Madsen AM, Tang D, Orjuela MA, Tsai WY, Garduno ER, Perera FP. Antioxidant vitamin supplementation reduces benzo(a)pyrene- DNA adducts and potential cancer risk in female smokers. Cancer Epidemiol Biomarkers and Prevention, (2005 );14(1):237-42
- Hassan NH, Fahmy MA, Farghaly AA, Hassan EE. Antimutagenic effect of selenium and vitamins against the genotoxicity induced by cobalt chloride in mice. Cytologia, (2006); 1(71): 213-222.
- Fahmy MA, Hassan NHA, Farghaly AA, Hassan ES. Studies on the genotoxic effect of beryllium chloride and the possible protective role of selenium/vitamins A, C and E. Mutation Research, (2008); 652(10): 103-111.
- Fishbane S, Wagner J. Sodium ferric gluconate complex in the treatment of iron deficiency for patients on dialysis. American Journal of Kidney Diseases, (2001); 37 (5): 879–83.
- Theil EC, Goss DJ. Living with Iron (and Oxygen): Questions and Answers about Iron Homeostasis. Chemical Reviews, (2009);109: 4568–4579.
- Schmid W. The micronucleus test. Mutation Research, (1975) ; 3(31): 9–15.
- Rahman A, Muktadir MG. SPSS: An imperative quantitative data analysis tool for social science research. International Journal of Research and Innovation in Social Science, (2021); 5(10): 300-2.
- Sommer S, Buraczewska I, Kruszewski M. Micronucleus Assay: The State of Art, and Future Directions. International Journal of Molecular Sciences, (2020); 21(4): 15-34.
- Fenech M. The in vitro micronucleus technique. Mutation Research, (2000); 20(2): 81-95.
- Terradas M, Martín M, Genescà A. Impaired nuclear functions in micronuclei results in genome instability and chromothripsis. Archives of Toxicology, (2016); 90(11): 2657-2667.
- El-Alfy NZI, Alqosaibi AI, Mahmoud MF, El-Ashry SR. An analysis of micronuclei and DNA damage induced by methotrexate treatment of male albino mice. The Egyptian journal of hospital medicine, (2016); 65(1): 504-514.
- Acar H, Caliskan U, Demirel S, Largaespada DA. Micronucleus incidence and their chromosomal origin related to therapy in acute lymphoblastic leukemia (ALL) patients: detection by micronucleus and FISH techniques. Teratogenesis, Carcinogenesis, and Mutagenesis, (2001); 21(5): 341-7.
- Maleek MI, Gheni DA. Cytogenetic effects of magnevist and methotrexate on mice bone marrow. Journal of Wasit for Science and Medicine, (2015); 8(2): 115-127.
- Novakovic T, Milocevic-Dordevic O, Grujicic D, Marinkovic D, Jankovic S, Arsenijevic S. Effect of intratumoral application of methotrexate in vivo on frequency of micronuclei in peripheral blood lymphocytes. Archive of Oncology, (2003); 11(1): 1-4.
- Rosenthal GJ, Weigand GW, Germolec DR .Suppression of B cell functions by methotrexate and trimethotrexate. Evidence for inhibition of purine biosynthesis as a major mechanism of action. The Journal of Immunology, (1988); 141(1): 410-416
- AL-Shaeli SJ, Ethaeb AM, Gharban HA. Determine the glucose regulatory role of decaffeinated Green Tea extract in reduces the metastasis and cell viability of MCF7 cell line. In AIP Conference Proceedings, (2022); 2394 (1): 1-8.
- Mayne S. Antioxidants nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. Journal of Nutrition, (2003); 133(15): 933-940.
- Hamed S, Al-Yhya NA, El-Khadragy MF, Al-Olayan EM, Alajmi RA, Hassan ZK. The protective properties of the strawberry (Fragaria ananassa) against carbon tetrachloride-induced hepatotoxicity in rats mediated by antiapoptotic and upregulation of antioxidant genes expression effects. Frontiers in Physiology, (2016); 7(10): 325-39.
- Wang J, Xiong K, Xu L, Zhang C, Zhao S, Liu Y. Dietary intake of vegetables and cooking oil was associated with drug-induced liver injury during tuberculosis treatment: a preliminary cohort study. Frontiers in Nutrition, (2021); 1(8): 652-671.
- Sahu K, Das RK. Reduction of clastogenic effect of clofazimine, an antileprosy drug, by vitamin A and vitamin C in bone marrow cells of mice. Food and chemical toxicology, (1994); 32(10): 911-915.
- Giri A, Khynriam D, Prasad SB. Vit. C mediated protection on cisplatin induced mutagenicity in mice. Mutation Research, (1998); 3 (2):139-48.
- Sampath M, Latha V, Prabhu V ,Saralaya V, Rajalakshmi R. A Comparison of Vitamin A and Leucovorin for the Prevention of Methotrexate-Induced Micronuclei Production in Rat Bone Marrow. Clinics, (2008); 63(6): 821–826.
- Ludwig H, Evstatiev R, Kornek G, Aapro M, Bauernhofer T, Buxhofer-Ausch V, Fridrik M, Geissler D, Geissler K, Gisslinger H, Koller E, Kopetzky G, Lang A, Rumpold H, Steurer M, Kamali H, Link H . Iron metabolism and iron supplementation in cancer patients. Wien Klin Wochenschr,(2015); 127(23-24): 907-19.
- Paul VD, Lill R. Biogenesis of cytosolic and nuclear iron-sulfur proteins and their role in genome stability. Biochimica et Biophysica Acta, (2015); 1853(6): 1528-39
- Puig S, Ramos-Alonso L, Romero AM, Martínez-Pastor MT. The elemental role of iron in DNA synthesis and repair. Metallomics, (2017); 9(11): 1483-1500.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0








